Document
... Which of the following statements concerning equilibrium is not true? A) A system that is disturbed from an equilibrium condition responds in a manner to restore equilibrium. B) Equilibrium in molecular systems is dynamic, with two opposing processes balancing one another. C) The value of the equili ...
... Which of the following statements concerning equilibrium is not true? A) A system that is disturbed from an equilibrium condition responds in a manner to restore equilibrium. B) Equilibrium in molecular systems is dynamic, with two opposing processes balancing one another. C) The value of the equili ...
Thermal Diffusion and Partial Molar Enthalpy Variations of n
... We shall describe the transport of heat and butane using zeolite as a frame of reference. These transport processes are interacting or “coupled” according to classical nonequilibrium thermodynamics;20 we shall find this theory appropriate. The theory defines the driving forces and fluxes in a system ...
... We shall describe the transport of heat and butane using zeolite as a frame of reference. These transport processes are interacting or “coupled” according to classical nonequilibrium thermodynamics;20 we shall find this theory appropriate. The theory defines the driving forces and fluxes in a system ...
+ H 2 (g) - UCF Chemistry
... • the part of the universe under study • the substances involved in the chemical and physical changes under investigation • in chemistry lab, the system may be the chemicals inside a beaker ...
... • the part of the universe under study • the substances involved in the chemical and physical changes under investigation • in chemistry lab, the system may be the chemicals inside a beaker ...
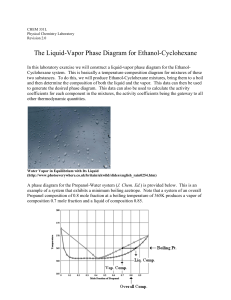
The Liquid-Vapor Phase Diagram for Ethanol
... condenser. Continue boiling until the temperature has become stable, that is, until it fluctuates about a mean value rather than showing an upward trend. It is quite useful to record temperature readings periodically as the system approaches a steady state, as this makes it easier to decide when thi ...
... condenser. Continue boiling until the temperature has become stable, that is, until it fluctuates about a mean value rather than showing an upward trend. It is quite useful to record temperature readings periodically as the system approaches a steady state, as this makes it easier to decide when thi ...
Handout - UNT Chemistry
... initial amount of reactant prior to dissociation, and neq is the amount of reactant present at equilibrium. Calculate for H2O gas at equilibrium at 2300 k and a total pressure of 1 bar. You may assume that << 1. Strategy: 1. Express number of moles of reactants and products in terms of . 2. Det ...
... initial amount of reactant prior to dissociation, and neq is the amount of reactant present at equilibrium. Calculate for H2O gas at equilibrium at 2300 k and a total pressure of 1 bar. You may assume that << 1. Strategy: 1. Express number of moles of reactants and products in terms of . 2. Det ...
Chemistry (306) - National Evaluation Series
... A. Valence electrons are raised to higher energy levels. B. Two or more types of atoms are combined. C. Energy is released to the surroundings. D. An element's atomic number is reduced. Correct Response and Explanation D. This question requires the examinee to demonstrate knowledge of the characteri ...
... A. Valence electrons are raised to higher energy levels. B. Two or more types of atoms are combined. C. Energy is released to the surroundings. D. An element's atomic number is reduced. Correct Response and Explanation D. This question requires the examinee to demonstrate knowledge of the characteri ...
Multi-Physics Interactions for Coupled Thermo-Electro
... The development of the MPIDs is a multi-disciplinary exercise: concepts from ...
... The development of the MPIDs is a multi-disciplinary exercise: concepts from ...
CHEM 1211 and CHEM 1212 National ACS Exams About the Exam
... formulas and techniques. Rather, it is a coherent set of knowledge that enables comprehension of the submicroscopic (chemical) world. As such, the ACS tests seek to uncover such genuine understanding. CHEM 1211 Example Questions There is an emphasis on conceptual questions. The actual exam wi ...
... formulas and techniques. Rather, it is a coherent set of knowledge that enables comprehension of the submicroscopic (chemical) world. As such, the ACS tests seek to uncover such genuine understanding. CHEM 1211 Example Questions There is an emphasis on conceptual questions. The actual exam wi ...
Table of contents
... ◦ Remember, as wavelength increases, frequency decreases (they are both inversely proportional). Therefore, gamma rays have the highest frequencies whereas radio waves have the lowest frequencies. Quantum Numbers ◦ Governing principles: ▪ Electrons are described as being in a state of rapid motion w ...
... ◦ Remember, as wavelength increases, frequency decreases (they are both inversely proportional). Therefore, gamma rays have the highest frequencies whereas radio waves have the lowest frequencies. Quantum Numbers ◦ Governing principles: ▪ Electrons are described as being in a state of rapid motion w ...
Model Test Papers
... (in ms-1) through a small hole on the side wall of the cylinder near its bottom is a) 10 b) 20 c)25 d) 5 19. A spring of force constant 800 N/m has an extension of 5 cm. The work done in extending it from 5cm to 15cm is a) 16 J b) 8 J c)32 J d)24 j 20. Two identical particles move towards each other ...
... (in ms-1) through a small hole on the side wall of the cylinder near its bottom is a) 10 b) 20 c)25 d) 5 19. A spring of force constant 800 N/m has an extension of 5 cm. The work done in extending it from 5cm to 15cm is a) 16 J b) 8 J c)32 J d)24 j 20. Two identical particles move towards each other ...
1 CHAPTER 8 HEAT CAPACITY, AND THE EXPANSION OF GASES
... raising the temperature. Consequently, more heat is required to raise the temperature of the gas by one degree if the gas is allowed to expand at constant pressure than if the gas is held at constant volume and not allowed to expand. Thus the heat capacity of a gas (or any substance for that matter) ...
... raising the temperature. Consequently, more heat is required to raise the temperature of the gas by one degree if the gas is allowed to expand at constant pressure than if the gas is held at constant volume and not allowed to expand. Thus the heat capacity of a gas (or any substance for that matter) ...
PHY 1150 - Concepts of Physics
... number of fundamental concepts of physics. It is designed to satisfy the needs of students who are interested in an overview of the concepts rather than a rigorous mathematical analysis of the topics as might be encountered in a traditional engineering level course in physics. Topics to be covered i ...
... number of fundamental concepts of physics. It is designed to satisfy the needs of students who are interested in an overview of the concepts rather than a rigorous mathematical analysis of the topics as might be encountered in a traditional engineering level course in physics. Topics to be covered i ...
Document
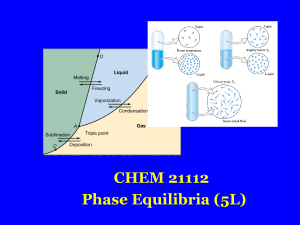
... • Liquids boil when the external pressure equals the vapor pressure. • Temperature of boiling point increases as pressure increases. • Two ways to get a liquid to boil: increase temperature or decrease pressure. – Pressure cookers operate at high pressure. At high pressure the boiling point of water ...
... • Liquids boil when the external pressure equals the vapor pressure. • Temperature of boiling point increases as pressure increases. • Two ways to get a liquid to boil: increase temperature or decrease pressure. – Pressure cookers operate at high pressure. At high pressure the boiling point of water ...