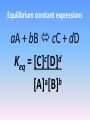* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chemical Equilibrium
Ionic liquid wikipedia , lookup
Marcus theory wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Acid–base reaction wikipedia , lookup
Detailed balance wikipedia , lookup
Electrochemistry wikipedia , lookup
Vapor–liquid equilibrium wikipedia , lookup
Ionic compound wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Thermodynamics wikipedia , lookup
Statistical mechanics wikipedia , lookup
Enzyme catalysis wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Industrial catalysts wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Reaction progress kinetic analysis wikipedia , lookup
Thermodynamic equilibrium wikipedia , lookup
Rate equation wikipedia , lookup
George S. Hammond wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Transition state theory wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Forward and reverse reactions taking place at equal rates It is a dynamic state - reactions are constantly occurring (a) Start: 10 goldfish in the left tank and 10 guppies in the right. (b) Equilibrium state. with 5 of each kind of fish in each tank. The equilibrium is dynamic; an averaged state and not a static condition . The fish do not stop swimming when they have become evenly mixed. (c) If we were to observe one single fish (here a guppy among goldfish). we would find that it spends half its time in each tank . Brightstorm videos Chemical Equilibrium Definition 5:01 http://www.youtube.com/watch?v=FYc_SoW2M40&list=PL06C3C4E3F 84C6A24&index=42 Crash course chemistry http://www.youtube.com/watch?v=g5wNg_dKsYY 10:56 Equilibrium http://www.youtube.com/watch?v=DP-vWN1yXrY 9:28 Equilibrium equations You don’t need to know how to do the RICE table starting at 4:40 Isaacs Teach http://www.youtube.com/watch?v=g4TKRInLdPA 10:09 Equilibrium Good basic explanation! http://www.youtube.com/watch?v=4z4_rc6nsKU 12:46 What is the equilibrium constant, Keq? Also very good explanation Equilibrium constant expressions aA + bB cC + dD c d Keq = [C] [D] a b [A] [B] General information about the Keq expression • Equilibrium [ ] of products are placed in the numerator. • Equilibrium [ ] of reactants are placed in the denominator. • Each [ ] term is raised to an exponent equal to its coefficient in the balanced equation. • If there is more than 1 product or reactant, the terms are multiplied. • Solids and liquids (pure substances) are not included in the Keq expression. This is because their [ ] are their densities. The density of a substance does not change with changing temperatures. • Keq is constant for a given reaction at a given temperature. There are no units associated with the value of Keq. • The value of Keq is independent of the: – individual [ ] of reactants and products – original [ ] of reactants and products – volume of the container. • The value of Keq is dependent on temperature. • What does the value of Keq tell you about a reaction? Keq >1: more products than reactants at equilibrium Keq < 1: more reactants than products at equilibrium Using equilibrium constants Calculating equilibrium concentrations: Example: At 1405 K, hydrogen sulfide, also called rotten egg gas (because of its bad odor), decomposes to form hydrogen and a diatomic sulfur molecule,S2. Keq = 2.27 x 10-3. (a) Write the balanced equation for the reaction described above. Write out the Keq expression. (b) Calculate the concentration of hydrogen gas if [S2] = 0.0540 M and [H2S] = 0.184 M. Solving the problem – part (a) 2H2S (g) 2H2 (g) + S2 (g) Keq = 2 [H2] [S2] 2 [H2S] Solution – (b) [H2]2= Keq [H2S]2 = [S2] (2.27 x 10-3)(0.184 M)2 = 0.0540 M [𝐻2]2 = 1.42 𝑥 10 − 3 𝑀 so [H2] = 3.77 x 10-2 M Le Châtelier’s principle: 1884 - Henri Le Châtelier When a stress is applied to a system at equilibrium, the system shifts in the direction that relieves the stress. Δ in concentration • Adding more of a reactant or product: the reaction will shift in the direction to consume a portion of what was added. – more reactant shifts right – more product shifts left • Removing some of a reactant or product: the reaction will shift in a direction to restore part of what was removed. – reactants removed reaction shifts left (i.e. the reverse reaction) – products removed reaction shifts right (i.e. the forward reaction). Δ in volume Relevant when discussing gaseous equilibria and when the number of moles of gaseous reactants differ from the number of moles of gaseous products. The change in volume is a result of a change in pressure of the gaseous system. • When P↓, the reaction will shift in a direction to↑ number of moles of gas. PCl5 (g) PCl3 (g) + Cl2 (g) 1 mol 2 mol 2NH3(g) N2(g) + 3H2(g) 2 mol 4 mol • When P↑, the reaction will shift in a direction to ↓ number of moles gas. PCl5 (g) PCl3 (g) + Cl2 (g) 1 mol 2 mol 2NH3(g) N2(g) + 3H2(g) 2 mol 4 mol Δ in temperature View changes in temperature as reactants or products. When the temperature of an equilibrium system is ↑ the reaction that is endothermic (ΔH>0) will take place. * forward rxn is endothermic more product (shifts to the right). * reverse rxn is endothermic less product (shifts to the left) When the temperature of an equilibrium system is ↓, the rxn which is exothermic (ΔH<0) will take place. * forward rxn is exothermic – more product (shifts to the right). * reverse rxn is exothermic – less product (shifts to the left) General rule: if the forward rxn is endothermic,↑K. If the forward rxn is exothermic ↓K. Animation demonstration http://www.learnerstv.com/animation/animation.php?ani=12 0&cat=chemistry This has some of the actual equilibria we will be investigating later this week. Le Chatelier’s Principle – Bozeman Science http://www.youtube.com/watch?v=PciV_Wuh9V8 7:00 good explanations with visuals and excellent discussion on how to increase yield of a reaction Equilibrium Disturbances – Bozeman Science http://www.youtube.com/watch?v=dd5p0VZ-MZg 5:36 This one will help you with the lab we’re doing. He also discusses the effect of disturbances (changes) in an equilibrium system and how they affect the value of K (the equilibrium constant) Reactions that go to completion Formation of a gas H2CO3 (aq) H2O (l) + CO2 (g) Formation of precipitate (remember Double displacement reactions) Formation of a slightly ionized product; often times H2O (i.e. in a neutralization reaction) H3O+ + OH- 2H2O (l) Solubility equilibria http://www.youtube.com/watch?v=YJdyEtB66A&feature=topics Brightstorm 4:17 The Solubility Product Constant, Ksp • Many important ionic compounds are only slightly soluble in water and equations are written to represent the equilibrium between the compound and the ions present in a saturated aqueous solution. • The solubility product constant, Ksp, is the product of the concentrations of the ions involved in a solubility equilibrium, each raised to a power equal to the stoichiometric coefficient of that ion in the chemical equation for the equilibrium. The Solubility Equilibrium Equation And Ksp CaF2 (s) Ca2+ (aq) + 2F- (aq) Ksp = [Ca2+][F-]2 Ksp = 5.3x10-9 As2S3 (s) 2As3+ (aq) + 3S2- (aq) Ksp = [As3+]2[S2-]3 Ksp = 6 x 10-51 Ksp And Molar Solubility • The solubility product constant is related to the solubility of an ionic solute, but Ksp and molar solubility - the molarity of a solute in a saturated aqueous solution - are not the same thing. • Calculating solubility equilibria fall into two categories: – determining a value of Ksp from experimental data – calculating equilibrium concentrations when Ksp is known. Calculating Ksp From Molar Solubility It is found that 1.2x10-3 mol of lead (II) iodide, PbI2, dissolves in 1.0 L of aqueous solution at 25 oC. What is the Ksp at this temperature? Solution: PbI2 (s) Pb2+ (aq) + 2I- (aq) Ksp = [Pb2+] [I-]2 Ksp = (1.2 x 10-3 M) (2 x 1.2 x 10-3 M)2 Ksp = 6.9 x10-9 Calculating Molar Solubility From Ksp Calculate the molar solubility of silver chromate, Ag2CrO4, in water from Ksp = 1.1x10-12 for Ag2CrO4. Solution: Ag2CrO4 (s) 2Ag+ (aq) + CrO4 2- (aq) Ksp = [Ag+]2 [CrO4 2-] Ksp = (2x)2(x) = 1.1 x 10-12 4x3 = 1.1 x 10-12 X = 6.5 x 10-5 M The Common Ion Effect In Solubility Equilibria • The common ion effect also affects solubility equilibria. • Le Châtelier’s principle is followed for the shift in concentration of products and reactants upon addition of either more products or more reactants to a solution. The solubility of a slightly soluble ionic compound is lowered when a second solute that furnishes a common ion is added to the solution. Solubility Equilibrium Calculation -The Common Ion Effect What is the solubility of Ag2CrO4 in 0.10 M K2CrO4? Ksp = 1.1x10-12 for Ag2CrO4. Ag2CrO4 (s) 2Ag+ (aq) + CrO4 2- (aq) Ksp = [Ag+]2 [CrO4 2-] Ksp = (2x)2(0.10) = 1.1 x 10-12 x = 1.65 x 10-6 M Comparison of solubility of Ag2CrO4 In pure water: 6.5 x 10-5 M (prior slide) In 0.10 M K2CrO4: 1.7 x 10-6 M The common ion effect!! Determining Whether Precipitation Occurs • Q is the ion product reaction quotient and is based on initial conditions of the reaction. • Q can then be compared to Ksp. • To predict if a precipitation occurs: - Precipitation should occur if Q > Ksp. - Precipitation cannot occur if Q < Ksp. - A solution is just saturated if Q = Ksp. DR lab: unexpected PPT according to solubility rules! Ca(OH)2 (s) Ca2+ (aq) + 2OH- (aq) Ksp = [Ca2+][OH-]2 Ksp = 6.5 x 10-6 Determining Whether Precipitation Occurs – An Example The concentration of calcium ion in blood plasma is 0.0025 M. If the concentration of oxalate ion is 1.0x10-7 M, do you expect calcium oxalate to precipitate? Ksp = 2.3x10-9. Three steps: (1) Determine the initial concentrations of ions. (2) Evaluate the reaction quotient Q. (3) Compare Q with Ksp. Solution CaC2O4 (s) Ca2+ (aq) + C2O42- (aq) Ksp = [Ca2+] [C2O42-] = 2.3x10-9 Qsp = (2.5 x 10-3 M) (1.0x10-7 M) = 2.5 x 10-10 2.5 x 10-10 < 2.3x10-9 Q < Ksp therefore no ppt will be formed Summary • The solubility product constant, Ksp, represents equilibrium between a slightly soluble ionic compound and its ions in a saturated aqueous solution. • The common ion effect is responsible for the reduction in solubility of a slightly soluble ionic compound. • The solubilities of some slightly soluble compounds depends strongly on pH. Equilibrium lab Fe(OH)3 (s) Fe3+ (aq) + 3OH- (aq) Ksp = [Fe3+][OH-]3 = 4 x 10-38 Q vs. Ksp Q = [Fe3+][OH-]3 = (0.2M)(6.0M)3 = 43.2 Q >Ksp so a PPT forms to take the Fe3+ out of solution Qualitative Inorganic Analysis • Acid-base chemistry, precipitation reactions, oxidation-reduction, and complex-ion formation all come into sharp focus in an area of analytical chemistry called classical qualitative inorganic analysis. • “Qualitative” signifies that the interest is in determining what is present, not how much is present. • Although classical qualitative analysis is not as widely used today as instrumental methods, it is still a good vehicle for applying all the basic concepts of equilibria in aqueous solutions.