Calculations In Chemistry Modules 8 to 10
... (hydrogen) has a mass close to one gram, simplifies the arithmetic in problems with other atoms, especially when we convert between grams and moles. That’s our goal: to calculate a count of particles by measuring their mass on a balance or scale. Counting the particles is important because in chemic ...
... (hydrogen) has a mass close to one gram, simplifies the arithmetic in problems with other atoms, especially when we convert between grams and moles. That’s our goal: to calculate a count of particles by measuring their mass on a balance or scale. Counting the particles is important because in chemic ...
Beverley John C. Beverley IE 500/PHI 598: Ontological Engineering
... foundations of the field of inquiry. It is with that in mind, and the lofty goals of terminological clarity, appropriate characterization of thermodynamic systems, and potential extensions into ...
... foundations of the field of inquiry. It is with that in mind, and the lofty goals of terminological clarity, appropriate characterization of thermodynamic systems, and potential extensions into ...
Homogeneous Catalysis
... the endpoint indicator. You might not have noticed, however, what happens when a solution that contains phenolphthalein in the presence of excess base is allowed to stand for a few minutes. Although the solution initially has a pink color, it gradually turns colorless as the phenolphthalein reacts w ...
... the endpoint indicator. You might not have noticed, however, what happens when a solution that contains phenolphthalein in the presence of excess base is allowed to stand for a few minutes. Although the solution initially has a pink color, it gradually turns colorless as the phenolphthalein reacts w ...
chemical kinetics
... Some reactions such as ionic reactions occur very fast, for example, precipitation of silver chloride occurs instantaneously by mixing of aqueous solutions of silver nitrate and sodium chloride. On the other hand, some reactions are very slow, for example, rusting of iron in the presence of air and ...
... Some reactions such as ionic reactions occur very fast, for example, precipitation of silver chloride occurs instantaneously by mixing of aqueous solutions of silver nitrate and sodium chloride. On the other hand, some reactions are very slow, for example, rusting of iron in the presence of air and ...
Kinetics and Reaction Pathways for Propane Dehydrogenation and
... The selectivity, product site-yield, and isotopic data presented are consistent with the propane conversion pathways shown in Scheme 1, which were also proposed on H-ZSM5 from kinetic and isotopic tracer studies.16 Propane undergoes initial dehydrogenation to form propene and H2 or cracking to form ...
... The selectivity, product site-yield, and isotopic data presented are consistent with the propane conversion pathways shown in Scheme 1, which were also proposed on H-ZSM5 from kinetic and isotopic tracer studies.16 Propane undergoes initial dehydrogenation to form propene and H2 or cracking to form ...
Now we turn to the study of chemical kinetics. Kinetics is the study of
... Note that simple or complex, all rate laws are functions of concentrations raised to some power, which can be an integer or a fraction, and constants. Some, but not all, reactions have rate laws that are functions of reactant concentrations alone and have a particularly simple form. If we write our ...
... Note that simple or complex, all rate laws are functions of concentrations raised to some power, which can be an integer or a fraction, and constants. Some, but not all, reactions have rate laws that are functions of reactant concentrations alone and have a particularly simple form. If we write our ...
18.3 Standard Entropies and the Third Law of
... nonspontaneous* describes a physical or chemical change that occurs only with the aid of an outside force or agent (18.2, introductory section) entropy (S) thermodynamic quantity that is a measure of how dispersed the energy of a system is among the different possible ways that system can contain en ...
... nonspontaneous* describes a physical or chemical change that occurs only with the aid of an outside force or agent (18.2, introductory section) entropy (S) thermodynamic quantity that is a measure of how dispersed the energy of a system is among the different possible ways that system can contain en ...
1 Fundamentals of Chemical Kinetics
... 1. The rate law might not have a simple form at all. 2. You might get tired before guessing the right form, and decide that one of the ones you have already done seems to fit the data “okay”, not realizing that another rate law (that you didn’t try) fits much better. If you use this method on a reac ...
... 1. The rate law might not have a simple form at all. 2. You might get tired before guessing the right form, and decide that one of the ones you have already done seems to fit the data “okay”, not realizing that another rate law (that you didn’t try) fits much better. If you use this method on a reac ...
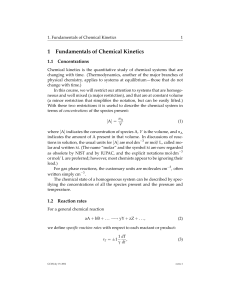
Chapter 12: Chemical Equilibrium • Chemical Equilibrium
... Equilibrium Constants • The amounts of reactants and products are determined using a mathematical model to describe equilibrium. – A relationship exists between reactant and product concentrations at equilibrium (the ratio of products to reactants is constant at a given temperature). ...
... Equilibrium Constants • The amounts of reactants and products are determined using a mathematical model to describe equilibrium. – A relationship exists between reactant and product concentrations at equilibrium (the ratio of products to reactants is constant at a given temperature). ...
ENTROPY
... In the second place, and more important, no on knows what entropy really is, so in a debate you will always have the advantage.”” Note that compound probabilities are multiplicative, uncertainties are additive and so is entropy. For equally-probable microstates totalising a number Ω, their probabili ...
... In the second place, and more important, no on knows what entropy really is, so in a debate you will always have the advantage.”” Note that compound probabilities are multiplicative, uncertainties are additive and so is entropy. For equally-probable microstates totalising a number Ω, their probabili ...
Unit 3 4 Balancing Chemical Reaction Equations by Inspection
... Before going on … I must introduce to you a little something called the polyatomic ions. (Review: recall that the terms ion ≠ ionic) B) Polyatomic ion: A chemically unstable group of bonded atoms, which end up with an unequal number of protons and electrons. Since most PAI are made of bonded nonmet ...
... Before going on … I must introduce to you a little something called the polyatomic ions. (Review: recall that the terms ion ≠ ionic) B) Polyatomic ion: A chemically unstable group of bonded atoms, which end up with an unequal number of protons and electrons. Since most PAI are made of bonded nonmet ...