Balancing Chemical Reactions
... common conventions, and some strategies that help simplify the process. Things That You Can’t Do When Balancing a Chemical Reaction. One of the most common mistakes when balancing a chemical reaction is to change the subscripts on compounds instead of changing the stoichiometric coefficients. For ex ...
... common conventions, and some strategies that help simplify the process. Things That You Can’t Do When Balancing a Chemical Reaction. One of the most common mistakes when balancing a chemical reaction is to change the subscripts on compounds instead of changing the stoichiometric coefficients. For ex ...
Final Exam - Seattle Central College
... CHEM 162: Final Exam Study Guide Chapter 16: Chemical Equilibrium equilibrium: state where the forward and reverse reactions or processes occur at the same rate – Know that concentrations are not changing at equilibrium, but they need not be equal to one another. – Be able to indicate when equilibri ...
... CHEM 162: Final Exam Study Guide Chapter 16: Chemical Equilibrium equilibrium: state where the forward and reverse reactions or processes occur at the same rate – Know that concentrations are not changing at equilibrium, but they need not be equal to one another. – Be able to indicate when equilibri ...
Unit 5 Notes
... titration, which produces a dark blue colour (this is caused by the reaction between starch and the remaining I2 in solution). After adding the starch (which acts as a more noticeable and therefore more precise indicator), the last of the S2O32- is added, causing the blue colour of the starch-I2 mix ...
... titration, which produces a dark blue colour (this is caused by the reaction between starch and the remaining I2 in solution). After adding the starch (which acts as a more noticeable and therefore more precise indicator), the last of the S2O32- is added, causing the blue colour of the starch-I2 mix ...
REDOX PowerPoint - Southmoreland School District
... it is bonded to metals in binary compounds. In these cases, its oxidation number is ___. (LiAlH4) 5. Group IA metals are ___, IIA metals are ___ and fluorine is always ___. 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is equal to ________________________. ...
... it is bonded to metals in binary compounds. In these cases, its oxidation number is ___. (LiAlH4) 5. Group IA metals are ___, IIA metals are ___ and fluorine is always ___. 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is equal to ________________________. ...
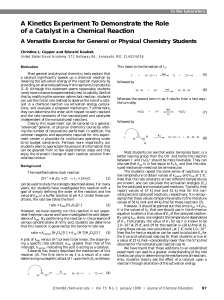
NIH Public Access - iGRAD
... Although constraints define a range of solutions, it is still possible to identify and analyze single points within the solution space. For example, we may be interested in identifying which point corresponds to the maximum growth rate or to maximum ATP production of an organism, given its particula ...
... Although constraints define a range of solutions, it is still possible to identify and analyze single points within the solution space. For example, we may be interested in identifying which point corresponds to the maximum growth rate or to maximum ATP production of an organism, given its particula ...
Equilibrium a.k.a. The Up Hill Climb
... E. Calculate change in concentration using coefficients or information in the problem. F. Solve for equilibrium concentrations. G. Substitute equilibrium concentrations into the K expression and calculate. H. Tricks: 1. Look for very small K values (where K < 10-5) , "x" may be negligible. You must ...
... E. Calculate change in concentration using coefficients or information in the problem. F. Solve for equilibrium concentrations. G. Substitute equilibrium concentrations into the K expression and calculate. H. Tricks: 1. Look for very small K values (where K < 10-5) , "x" may be negligible. You must ...
Torque
... torques. Experimental Procedure and Data Analysis A. Torques 1. Adjust the location of the fulcrum so that the meter stick, with no weights hanging on it, is in static equilibrium (balance) in a horizontal position. The position of the fulcrum is roughly the center of gravity of the meter stick. Not ...
... torques. Experimental Procedure and Data Analysis A. Torques 1. Adjust the location of the fulcrum so that the meter stick, with no weights hanging on it, is in static equilibrium (balance) in a horizontal position. The position of the fulcrum is roughly the center of gravity of the meter stick. Not ...
F325 How Far How Fast test
... units, if any ........................................................................................... ...
... units, if any ........................................................................................... ...