Condensed Matter 2
... all reactants, the reaction is not complete, and the reaction is reversible. A + B = C + D In principle, all chemical reactions are reversible, but this reversibility may not be observable if the fraction of products in the equilibrium mixture is very small, or if the reverse reaction is kinetic ...
... all reactants, the reaction is not complete, and the reaction is reversible. A + B = C + D In principle, all chemical reactions are reversible, but this reversibility may not be observable if the fraction of products in the equilibrium mixture is very small, or if the reverse reaction is kinetic ...
Wk2_Monday
... The empirical formula tells you the simplest ratio of the individual elements in the compound. For an ionic compound this information is enough. For a molecular compound this may not be enough since the empirical formula may not be the molecular formula. Knowledge of the MOLAR MASS of the compound a ...
... The empirical formula tells you the simplest ratio of the individual elements in the compound. For an ionic compound this information is enough. For a molecular compound this may not be enough since the empirical formula may not be the molecular formula. Knowledge of the MOLAR MASS of the compound a ...
AP Syllabus 95-96 - Bremen High School District 228
... AP courses are college level courses offered in a high school setting. This requires some compromise in the handling of routine matters, such as attendance and makeup work. Stated below are certain policies for this course. Some flexibility may apply in special circumstances and students are encoura ...
... AP courses are college level courses offered in a high school setting. This requires some compromise in the handling of routine matters, such as attendance and makeup work. Stated below are certain policies for this course. Some flexibility may apply in special circumstances and students are encoura ...
dx cx dx and x - Cameron University
... 16.10 Relate entropy to the reversibility and irreversibility of a process ...
... 16.10 Relate entropy to the reversibility and irreversibility of a process ...
AOSS_401_20070924_L08_Static_Wave_Thermo
... This is the temperature a parcel would have if it was moved from some pressure and temperature to the surface. This is Poisson’s equation. ...
... This is the temperature a parcel would have if it was moved from some pressure and temperature to the surface. This is Poisson’s equation. ...
CHEM 5142
... experimental results for the Fe(aq)2+/3+ electron exchange rate, k22, and the calculated value obtained from cross reactions by the Marcus theory? c) What is the role of the sulfate ion in this reaction? VII. Activation, Addition and Insertion Reactions and Catalysis. ...
... experimental results for the Fe(aq)2+/3+ electron exchange rate, k22, and the calculated value obtained from cross reactions by the Marcus theory? c) What is the role of the sulfate ion in this reaction? VII. Activation, Addition and Insertion Reactions and Catalysis. ...
Topic 5 - Chemical Reactions
... Reaction rates/kinetics are affected by activation energy, catalysis, and the degree of randomness (entropy). Catalysts decrease the amount of activation energy needed. Chemical reactions based on the net heat energy are exothermic reactions (heat producing) and endothermic reactions (heat absorbing ...
... Reaction rates/kinetics are affected by activation energy, catalysis, and the degree of randomness (entropy). Catalysts decrease the amount of activation energy needed. Chemical reactions based on the net heat energy are exothermic reactions (heat producing) and endothermic reactions (heat absorbing ...
Presentation by class of 2013
... concentration of water must be included even if it is in the liquid phase; as the concentration of water can vary. However, the concentration of water must be included if it is in the gas phase. State symbols dictate when to take the products or reactants into account when calculating the equilibr ...
... concentration of water must be included even if it is in the liquid phase; as the concentration of water can vary. However, the concentration of water must be included if it is in the gas phase. State symbols dictate when to take the products or reactants into account when calculating the equilibr ...
HUGPS268 (acidic and basic solutions)
... is not synonymous with the charge on that atom, so a change in oxidation number does not require a change in actual charge. Take the example of the following half-reaction: H2C2O4(aq) : 2CO2(g) ⫹ 2H⫹(aq) ⫹ 2e⫺ Carbon changes oxidation state from ⫹3 to ⫹4. Carbon is oxidized even though it is not ion ...
... is not synonymous with the charge on that atom, so a change in oxidation number does not require a change in actual charge. Take the example of the following half-reaction: H2C2O4(aq) : 2CO2(g) ⫹ 2H⫹(aq) ⫹ 2e⫺ Carbon changes oxidation state from ⫹3 to ⫹4. Carbon is oxidized even though it is not ion ...
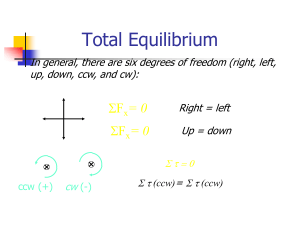
5. STATIC EQUILIBRIUM. Key words: Static Equilibrium, First
... Where r is the distance from the axis of rotation to the point where force F applied; F is the magnitude of the applied force; θ is the angle between the line of action of the force and a line connecting axis of rotation and the point at which the force is applied (see Fig. 5.1). Usually we will use ...
... Where r is the distance from the axis of rotation to the point where force F applied; F is the magnitude of the applied force; θ is the angle between the line of action of the force and a line connecting axis of rotation and the point at which the force is applied (see Fig. 5.1). Usually we will use ...