Laboratories to be performed
... kinetics, chemical equilibrium, and thermodynamics. This course aims to: 1) provide the students with a basic understanding of chemical concepts in a manner that is equivalent to a first-year college chemistry course; 2) provide students with a safe and enjoyable learning environment where they feel ...
... kinetics, chemical equilibrium, and thermodynamics. This course aims to: 1) provide the students with a basic understanding of chemical concepts in a manner that is equivalent to a first-year college chemistry course; 2) provide students with a safe and enjoyable learning environment where they feel ...
ggh - Library
... metal loadings, catalyst calcination temperatures and reaction temperatures. The results showed that the Pd catalyst prepared by impregnation favoured the hydrogenation of the conjugated double bond of citral, giving citronellal as the primary hydrogenation products, whereas the amounts of unsaturat ...
... metal loadings, catalyst calcination temperatures and reaction temperatures. The results showed that the Pd catalyst prepared by impregnation favoured the hydrogenation of the conjugated double bond of citral, giving citronellal as the primary hydrogenation products, whereas the amounts of unsaturat ...
PFC ,RR-86-1 The Kinetics Of Liquid Lithium Reaction With Oxygen
... lithium spill. Furthermore, lithium reaction kinetics experiments have also been conducted at MIT. In 1984, W. Ijams performed a series of experiments in order to obtain the lithium-nitrogen reaction rate as a function of the lithium pool temperature. These experiments have been performed to increas ...
... lithium spill. Furthermore, lithium reaction kinetics experiments have also been conducted at MIT. In 1984, W. Ijams performed a series of experiments in order to obtain the lithium-nitrogen reaction rate as a function of the lithium pool temperature. These experiments have been performed to increas ...
Molecular Dynamics Simulations of a Lesion in DNA
... repair and the formation mechanisms of the predominant UV-induced lesion in DNA, namely the thymine dimer. By disrupting the function of DNA, this lesion can trigger complex biological responses, including apoptosis, immune suppression, and carcinogenesis [1]. Here, we will first provide some insigh ...
... repair and the formation mechanisms of the predominant UV-induced lesion in DNA, namely the thymine dimer. By disrupting the function of DNA, this lesion can trigger complex biological responses, including apoptosis, immune suppression, and carcinogenesis [1]. Here, we will first provide some insigh ...
Matrix Isolation Spectroscopic and Theoretical Study of Carbon
... cations form adducts with CO2.1−6 Matrix isolation spectroscopic as well as gas phase kinetic investigations have been performed on the reactions of neutral transition metal atoms with CO2, which indicated that the ground state early transition metal atoms were able to activate the CO bond of CO2 i ...
... cations form adducts with CO2.1−6 Matrix isolation spectroscopic as well as gas phase kinetic investigations have been performed on the reactions of neutral transition metal atoms with CO2, which indicated that the ground state early transition metal atoms were able to activate the CO bond of CO2 i ...
CATALYTIC ACTIVITY OF BINARY AND TRIPLE SYSTEMS BASED
... complexes to promote a variety of reactions involving the transfer of electrons. It was reported that rates of O2 reduction by MnII complex are accelerated in the presence of group 2 metal ions [8]. This effect is typified in metalloproteins such as the copper zinc superoxide dismutase, in which bot ...
... complexes to promote a variety of reactions involving the transfer of electrons. It was reported that rates of O2 reduction by MnII complex are accelerated in the presence of group 2 metal ions [8]. This effect is typified in metalloproteins such as the copper zinc superoxide dismutase, in which bot ...
grafted chitosan - Repositorio Académico
... from a set of preliminary tests with various KPS/FAS compositions and then by studying separately the effect of the KPS concentration and its combination with different FAS concentrations. In this way, by keeping all other reaction variables constant, the amount of KPS used was varied between 10–4 a ...
... from a set of preliminary tests with various KPS/FAS compositions and then by studying separately the effect of the KPS concentration and its combination with different FAS concentrations. In this way, by keeping all other reaction variables constant, the amount of KPS used was varied between 10–4 a ...
THE WEATHERING OF SULFIDE-BEARING ROCKS ASSOCIATED
... data serve as the major reference for evaluation of the simulations of this study. Jarosite precipitation also indicates that elements from both gangue (K,Na) and sulfide (Fe,S) minerals are important in the weather ing reactions. Additional evidence for chemical reaction between acid sulfate groun ...
... data serve as the major reference for evaluation of the simulations of this study. Jarosite precipitation also indicates that elements from both gangue (K,Na) and sulfide (Fe,S) minerals are important in the weather ing reactions. Additional evidence for chemical reaction between acid sulfate groun ...
Phylogenetic Classification of Protozoa Based on the
... monomer. All residues from 3 to 521 are clearly defined in the electron density, allowing the entire protein model to be visualized. The bulk of the DHFR domains do not contact each other, but the linker polypeptide between the DHFR and TS domains crosses from one DHFR monomer (A) to the other DHFR ...
... monomer. All residues from 3 to 521 are clearly defined in the electron density, allowing the entire protein model to be visualized. The bulk of the DHFR domains do not contact each other, but the linker polypeptide between the DHFR and TS domains crosses from one DHFR monomer (A) to the other DHFR ...
Phylogenetic Classification of Protozoa Based on the Structure of
... monomer. All residues from 3 to 521 are clearly defined in the electron density, allowing the entire protein model to be visualized. The bulk of the DHFR domains do not contact each other, but the linker polypeptide between the DHFR and TS domains crosses from one DHFR monomer (A) to the other DHFR ...
... monomer. All residues from 3 to 521 are clearly defined in the electron density, allowing the entire protein model to be visualized. The bulk of the DHFR domains do not contact each other, but the linker polypeptide between the DHFR and TS domains crosses from one DHFR monomer (A) to the other DHFR ...
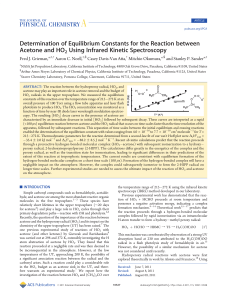
1. (a) (i) 2Ca(NO3)2 → 2CaO + 4NO2 + O2 formulae correct (1
... • Recognizing the existence of hydrogen bonds ( between molecules) (1) • That each molecule can form more than one hydrogen bond because of the two OH (and two S=O groups) / or a description of hydrogen bonds in this case / or a diagram showing the hydrogen bonds (1) ...
... • Recognizing the existence of hydrogen bonds ( between molecules) (1) • That each molecule can form more than one hydrogen bond because of the two OH (and two S=O groups) / or a description of hydrogen bonds in this case / or a diagram showing the hydrogen bonds (1) ...
Oxidation of Reduced Sulfur Species: Carbon
... O is 2.2 × 1013 s−1 at the high-pressure limit, 6.7 times smaller than that for dissociation back to CS + O2. This would suggest that above ∼1400 K it is the second barrier that becomes rate limiting. However, because the cis pathway appears to have no barrier, in fact rapid rotation allows for fast ...
... O is 2.2 × 1013 s−1 at the high-pressure limit, 6.7 times smaller than that for dissociation back to CS + O2. This would suggest that above ∼1400 K it is the second barrier that becomes rate limiting. However, because the cis pathway appears to have no barrier, in fact rapid rotation allows for fast ...
Kinetics and Reaction Pathways for Propane Dehydrogenation and
... predominantly to ethane. Therefore, it appears that ethene is mainly consumed via hydrogenation reactions to form ethane, although some ethene undergoes oligomerization with other alkenes and ultimately leads to higher alkenes and aromatics. The 13C content and the isotopomer distribution in product ...
... predominantly to ethane. Therefore, it appears that ethene is mainly consumed via hydrogenation reactions to form ethane, although some ethene undergoes oligomerization with other alkenes and ultimately leads to higher alkenes and aromatics. The 13C content and the isotopomer distribution in product ...
A Propagation of Error Analysis of the Enzyme Activity Expression. A
... module. CVv, CVv represent the uncertainty of volume measurement ofthe sample and reagentpipetting system.C Vtmjn istheuncertaintyoftime when theactualabsorbancemeasurements are made by the computer or other timing device. For a computer, the uncertainty is of the order of 10 ms or less. The magnitu ...
... module. CVv, CVv represent the uncertainty of volume measurement ofthe sample and reagentpipetting system.C Vtmjn istheuncertaintyoftime when theactualabsorbancemeasurements are made by the computer or other timing device. For a computer, the uncertainty is of the order of 10 ms or less. The magnitu ...
Enzyme catalysis

Enzyme catalysis is the increase in the rate of a chemical reaction by the active site of a protein. The protein catalyst (enzyme) may be part of a multi-subunit complex, and/or may transiently or permanently associate with a Cofactor (e.g. adenosine triphosphate). Catalysis of biochemical reactions in the cell is vital due to the very low reaction rates of the uncatalysed reactions. A key driver of protein evolution is the optimization of such catalytic activities via protein dynamics.The mechanism of enzyme catalysis is similar in principle to other types of chemical catalysis. By providing an alternative reaction route the enzyme reduces the energy required to reach the highest energy transition state of the reaction. The reduction of activation energy (Ea) increases the amount of reactant molecules that achieve a sufficient level of energy, such that they reach the activation energy and form the product. As with other catalysts, the enzyme is not consumed during the reaction (as a substrate is) but is recycled such that a single enzyme performs many rounds of catalysis.