Laboratories to be performed
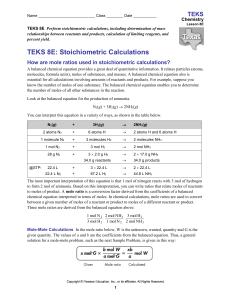
... 13. Solve stoichiometry problems for reactions between substances that are in solution. (Sect. 4.3) Prob: 113 14. Describe how a titrations is performed, and carry out calculations related to titrations. (Sect. 4.6) Prob: 76 b, 77, 83, 92, 102 Laboratories to be performed: 1. Determination of the co ...
... 13. Solve stoichiometry problems for reactions between substances that are in solution. (Sect. 4.3) Prob: 113 14. Describe how a titrations is performed, and carry out calculations related to titrations. (Sect. 4.6) Prob: 76 b, 77, 83, 92, 102 Laboratories to be performed: 1. Determination of the co ...
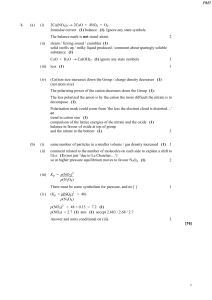
1. (a) (i) 2Ca(NO3)2 → 2CaO + 4NO2 + O2 formulae correct (1
... Either compromise in which the rate is more important than the position of equilibrium or optimum temperature for catalyst to operate or ...
... Either compromise in which the rate is more important than the position of equilibrium or optimum temperature for catalyst to operate or ...