Dynamic Equilibria in Solvent-Mediated Anion, Cation and Ligand
... evidence is given for such a mechanism or assignment. Compared to typical molecular complexes of low to medium molecular weights, many coordination polymers have a perceived low solubility in most conventional solvents and so are often described as “insoluble”.[17, 18, 29] In addition, it has been ...
... evidence is given for such a mechanism or assignment. Compared to typical molecular complexes of low to medium molecular weights, many coordination polymers have a perceived low solubility in most conventional solvents and so are often described as “insoluble”.[17, 18, 29] In addition, it has been ...
A thermodynamic model for the prediction of phase equilibria and
... 1987; Pruess and Spycher, 2007; Li et al., 2007). Many researchers have also tried to establish thermodynamic models for this system. For example, Harvie et al. (1984) used Pitzer model to predict mineral solubility in the system Na–K–Mg–Ca–H–Cl-SO4–OH–HCO3–CO3–CO2–H2O. The model has good precision, ...
... 1987; Pruess and Spycher, 2007; Li et al., 2007). Many researchers have also tried to establish thermodynamic models for this system. For example, Harvie et al. (1984) used Pitzer model to predict mineral solubility in the system Na–K–Mg–Ca–H–Cl-SO4–OH–HCO3–CO3–CO2–H2O. The model has good precision, ...
Influence of Ionic Mobile Phase Additives with Low Charge
... chromatography is the separation and identification of mixtures of ionizable and ionic analytes. Since the protonation of these compounds is pH dependent, the use of reversed phase-high performance liquid chromatography (RP-HPLC) is restricted to the use of mobile phases in which the pH of the aqueo ...
... chromatography is the separation and identification of mixtures of ionizable and ionic analytes. Since the protonation of these compounds is pH dependent, the use of reversed phase-high performance liquid chromatography (RP-HPLC) is restricted to the use of mobile phases in which the pH of the aqueo ...
Fulltext: english,
... electrolytes from one solvent to another.62−66 Even more relevant are data for single-ion values.67,68 Although these are based on an extra-thermodynamic convention it allows selecting the appropriate solvent to carry out the experimental work, particularly if the solvation properties of the recepto ...
... electrolytes from one solvent to another.62−66 Even more relevant are data for single-ion values.67,68 Although these are based on an extra-thermodynamic convention it allows selecting the appropriate solvent to carry out the experimental work, particularly if the solvation properties of the recepto ...
Solubility
... concentrations are equal to the real concentrations of species when the species behave under ideal conditions. For ionic species, ideal conditions are “dilute” conditions, where each molecule or ion behaves independently. At higher concentrations, the species in solution do not necessarily behave in ...
... concentrations are equal to the real concentrations of species when the species behave under ideal conditions. For ionic species, ideal conditions are “dilute” conditions, where each molecule or ion behaves independently. At higher concentrations, the species in solution do not necessarily behave in ...
International Journal of Mass Spectrometry Triacetone triperoxide
... has no application in civil engineering or in military. However, TATP can be easily synthesized with readily available chemical precursors such as acetone, hydrogen peroxide and various acid catalysts, thus it has increasingly become an improvised explosive material frequently used in the terrorism ...
... has no application in civil engineering or in military. However, TATP can be easily synthesized with readily available chemical precursors such as acetone, hydrogen peroxide and various acid catalysts, thus it has increasingly become an improvised explosive material frequently used in the terrorism ...
Soluble - HCC Learning Web
... Solubility Equilibria and the Solubility Product Solubility Rules Soluble: Dissolve - Do NOT form a solid precipitate. 1.alkali metal ions and ammonium ion: Li+, Na+, K+, NH4+ 2.acetate ion: C2H3O213.nitrate ion: NO314.halide ions (X): Cl-, Br-, I- (Exceptions: AgX, HgX, and PbX2 are insoluble) 5.su ...
... Solubility Equilibria and the Solubility Product Solubility Rules Soluble: Dissolve - Do NOT form a solid precipitate. 1.alkali metal ions and ammonium ion: Li+, Na+, K+, NH4+ 2.acetate ion: C2H3O213.nitrate ion: NO314.halide ions (X): Cl-, Br-, I- (Exceptions: AgX, HgX, and PbX2 are insoluble) 5.su ...
cmc by uv 2
... Surfactants, sometimes called surface-active agents or detergents, are among the most versatile chemicals available. They have applications in many areas, including chemistry (chemical kinetics or equilibria), biology (as membrane mimetics), and pharmacy (1). Surfactants are amphiphilic materials co ...
... Surfactants, sometimes called surface-active agents or detergents, are among the most versatile chemicals available. They have applications in many areas, including chemistry (chemical kinetics or equilibria), biology (as membrane mimetics), and pharmacy (1). Surfactants are amphiphilic materials co ...
TRIPURA UNIVERSITY SURYAMANINAGAR SYLLABUS FOR B.Sc THREE-YEAR DEGREE (GENERAL AND HONOURS) COURSE
... both) questions of 1 mark each from three units, of which five are to be answered. f) Two questions of 15 marks each are to be set from each unit, out of which one question is to be answered . Each question of 15 marks may be divided into three or more parts having a maximum of 8 marks for a part. U ...
... both) questions of 1 mark each from three units, of which five are to be answered. f) Two questions of 15 marks each are to be set from each unit, out of which one question is to be answered . Each question of 15 marks may be divided into three or more parts having a maximum of 8 marks for a part. U ...
Solubility Workbook
... The following workbook is designed to ensure that you can demonstrate your understanding of all aspects of the solubility unit. Ask yourself, “do I want to do well in this class?” If you are determined to be successful the minimum expectation that you should have for yourself is that you do all of t ...
... The following workbook is designed to ensure that you can demonstrate your understanding of all aspects of the solubility unit. Ask yourself, “do I want to do well in this class?” If you are determined to be successful the minimum expectation that you should have for yourself is that you do all of t ...
Thermal transport properties of halide solid solutions: Experiments
... This temperature is the safest maximum temperature at which the flash apparatus can be used without damage by liquid formation. On the other hand, the prediction of the thermal conductivity by our EMD is limited to rather high temperatures for practical reasons discussed below. As also shown in Figu ...
... This temperature is the safest maximum temperature at which the flash apparatus can be used without damage by liquid formation. On the other hand, the prediction of the thermal conductivity by our EMD is limited to rather high temperatures for practical reasons discussed below. As also shown in Figu ...
Hidden Scale Invariance in Condensed Matter
... fact that liquid states can be continuously converted into gas states.7 The van der Waals equation emphasizes the liquid−gas resemblance. However, density and specific heat of liquids at ambient conditions are typically close to those of the corresponding crystal, not to their gas values. Back in the ...
... fact that liquid states can be continuously converted into gas states.7 The van der Waals equation emphasizes the liquid−gas resemblance. However, density and specific heat of liquids at ambient conditions are typically close to those of the corresponding crystal, not to their gas values. Back in the ...
Enthalpy change - Don`t Trust Atoms
... • This is the temperature at which the reaction is just feasible. • In a closed system an equilibrium between products and reactants ...
... • This is the temperature at which the reaction is just feasible. • In a closed system an equilibrium between products and reactants ...
Kinetics of crystal nucleation in ionic solutions
... IS = 0.09 M. At a given X and IS the respective size of barite crystals decreased with increasing b2 in chloride salts of different cations and remained constant in sodium salts of different anions. We suggest that ionic salts affect the kinetics of barite nucleation and growth due to their influence on ...
... IS = 0.09 M. At a given X and IS the respective size of barite crystals decreased with increasing b2 in chloride salts of different cations and remained constant in sodium salts of different anions. We suggest that ionic salts affect the kinetics of barite nucleation and growth due to their influence on ...
Decrease = stress More Fe(OH) 2 dissolves in response Solubility
... (CaCO3) forms caves • Note: Limestone is water “insoluble” (How can this be?) – Precipitation of limestone (CaCO3) forms stalactites and stalagmites in underground caverns – Precipitation of insoluble Ca3(PO4)2 and/or CaC2O4 in the kidneys forms kidney stones – Dissolving of tooth enamel, Ca5(PO4)3O ...
... (CaCO3) forms caves • Note: Limestone is water “insoluble” (How can this be?) – Precipitation of limestone (CaCO3) forms stalactites and stalagmites in underground caverns – Precipitation of insoluble Ca3(PO4)2 and/or CaC2O4 in the kidneys forms kidney stones – Dissolving of tooth enamel, Ca5(PO4)3O ...
Chemical fractionation at environmental interfaces
... Interfaces play important roles in many physical, chemical, and biological processes, many of which have important environmental implications. In this thesis, I present three independent studies on the fractionation of chemicals at environmental interfaces. The first part describes anion fractionati ...
... Interfaces play important roles in many physical, chemical, and biological processes, many of which have important environmental implications. In this thesis, I present three independent studies on the fractionation of chemicals at environmental interfaces. The first part describes anion fractionati ...
Lecture 11 Notes
... Example: The boiling point of N2(l) is -195.81 oC. The boiling point of O2(l) is -182.96 oC. If only dispersion forces are considered, one would predict the boiling point of NO(l) to be in between that of O2(l) and N2(l) . The actual boiling point of NO(l) is -151.76 oC, much higher than either that ...
... Example: The boiling point of N2(l) is -195.81 oC. The boiling point of O2(l) is -182.96 oC. If only dispersion forces are considered, one would predict the boiling point of NO(l) to be in between that of O2(l) and N2(l) . The actual boiling point of NO(l) is -151.76 oC, much higher than either that ...
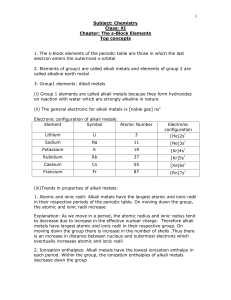
The s-Block Elements Top concepts 1. The s-block
... therefore, the electrons are attracted more towards the nucleus. As a result, their atomic and ionic radii are smaller than those of alkali metals. Atomic and ionic radii increase on moving down the group, due to screening effect and increases in the number of the shells. 2. Ionisation enthalpies: A ...
... therefore, the electrons are attracted more towards the nucleus. As a result, their atomic and ionic radii are smaller than those of alkali metals. Atomic and ionic radii increase on moving down the group, due to screening effect and increases in the number of the shells. 2. Ionisation enthalpies: A ...
Ksp - ChemConnections
... separate the metals as their iodides. Kspof AgI = 8.3 x 10-17; Kspof CuI = 1.0 x 10-12. Plan: Since the two iodides have the same formula type (1:1), directly compare their Ksp values. NOTE: CuI is about 100,000 times more soluble than AgI. Therefore, AgI precipitates first. Solve for [I -], which ...
... separate the metals as their iodides. Kspof AgI = 8.3 x 10-17; Kspof CuI = 1.0 x 10-12. Plan: Since the two iodides have the same formula type (1:1), directly compare their Ksp values. NOTE: CuI is about 100,000 times more soluble than AgI. Therefore, AgI precipitates first. Solve for [I -], which ...
Kinetics of the fading of phenolphthalein in alkaline solution
... of no consequence to the titration, and the solution is discarded without further thought. Yet this fading of phenolphthalein in alkaline solution is interesting in its own right and can serve as the basis for an experiment illustrating pseudo-first-order kinetics. The procedure is extremely simple ...
... of no consequence to the titration, and the solution is discarded without further thought. Yet this fading of phenolphthalein in alkaline solution is interesting in its own right and can serve as the basis for an experiment illustrating pseudo-first-order kinetics. The procedure is extremely simple ...
EFFECTS OF THE CHANGES IN SLOPE OCCURRING ON
... Liquidus and solidus paths in the system diopside-anorthite-albite exhibit changes in slope. When plagioclase crystallizes alone from a ternary liquid both paths are steeper than in the system anorthite-albite, but when plagioclase is joined by diopside both paths become much less step than in the b ...
... Liquidus and solidus paths in the system diopside-anorthite-albite exhibit changes in slope. When plagioclase crystallizes alone from a ternary liquid both paths are steeper than in the system anorthite-albite, but when plagioclase is joined by diopside both paths become much less step than in the b ...
IJEMS 3(6) 243-247
... has been found to increase than that in absence of such redox couples or complexing agents. Although a considerable amount of studies have been carried out on the dissolution of hematite in the presence and absence of reductants, no report on dissolution using both organic and inorganic reductants a ...
... has been found to increase than that in absence of such redox couples or complexing agents. Although a considerable amount of studies have been carried out on the dissolution of hematite in the presence and absence of reductants, no report on dissolution using both organic and inorganic reductants a ...
High-Pressure Solubility Data of Methane in Aniline
... The application of high pressures to industrial processes has led to engineering operations that frequently require knowledge of solubilities of gases in liquids at pressures higher than those for which such data are ordinarily available. The production of aniline by reduction of nitrobenzene is suc ...
... The application of high pressures to industrial processes has led to engineering operations that frequently require knowledge of solubilities of gases in liquids at pressures higher than those for which such data are ordinarily available. The production of aniline by reduction of nitrobenzene is suc ...
Chemical Equilibrium
... The Solubility Product Constant, Ksp • Many important ionic compounds are only slightly soluble in water and equations are written to represent the equilibrium between the compound and the ions present in a saturated aqueous solution. • The solubility product constant, Ksp, is the product of the co ...
... The Solubility Product Constant, Ksp • Many important ionic compounds are only slightly soluble in water and equations are written to represent the equilibrium between the compound and the ions present in a saturated aqueous solution. • The solubility product constant, Ksp, is the product of the co ...
Ionic liquid
An ionic liquid (IL) is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below some arbitrary temperature, such as 100 °C (212 °F). While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ionic liquids are largely made of ions and short-lived ion pairs. These substances are variously called liquid electrolytes, ionic melts, ionic fluids, fused salts, liquid salts, or ionic glasses. Ionic liquids have many applications, such as powerful solvents and electrically conducting fluids (electrolytes). Salts that are liquid at near-ambient temperature are important for electric battery applications, and have been used as sealants due to their very low vapor pressure.Any salt that melts without decomposing or vaporizing usually yields an ionic liquid. Sodium chloride (NaCl), for example, melts at 801 °C (1,474 °F) into a liquid that consists largely of sodium cations (Na+) and chloride anions (Cl−). Conversely, when an ionic liquid is cooled, it often forms an ionic solid—which may be either crystalline or glassy.The ionic bond is usually stronger than the Van der Waals forces between the molecules of ordinary liquids. For that reason, common salts tend to melt at higher temperatures than other solid molecules. Some salts are liquid at or below room temperature. Examples include compounds based on the 1-Ethyl-3-methylimidazolium (EMIM) cation and include: EMIM:Cl, EMIM dicyanamide, (C2H5)(CH3)C3H3N+2·N(CN)−2, that melts at −21 °C (−6 °F); and 1-butyl-3,5-dimethylpyridinium bromide which becomes a glass below −24 °C (−11 °F).Low-temperature ionic liquids can be compared to ionic solutions, liquids that contain both ions and neutral molecules, and in particular to the so-called deep eutectic solvents, mixtures of ionic and non-ionic solid substances which have much lower melting points than the pure compounds. Certain mixtures of nitrate salts can have melting points below 100 °C.The term ""ionic liquid"" in the general sense was used as early as 1943.