Alkenes 4 - ChemWeb (UCC)
... Long before anything was known about the mechanism of this reaction it was recognised that 'Addition of HX to an alkene will proceed in such a way as to attach hydrogen to the least substituted carbon and X to the most substituted carbon'. This is known as Markovnikov's Rule after the Russian chemis ...
... Long before anything was known about the mechanism of this reaction it was recognised that 'Addition of HX to an alkene will proceed in such a way as to attach hydrogen to the least substituted carbon and X to the most substituted carbon'. This is known as Markovnikov's Rule after the Russian chemis ...
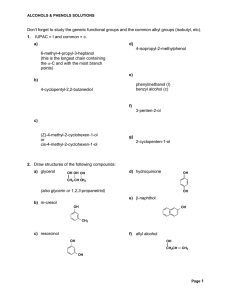
Don`t forget to study the generic functional groups and the common
... allows hydrogenation of H2/Rh an aromatic ring at standard pressure and temperature. Carboxylic acids are not reduced by hydrogen. ...
... allows hydrogenation of H2/Rh an aromatic ring at standard pressure and temperature. Carboxylic acids are not reduced by hydrogen. ...
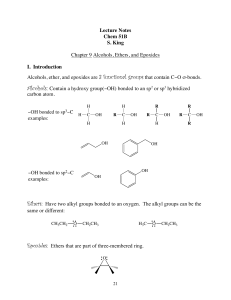
Alcohols, Ethers, and Epoxides
... Compounds with two hydroxy groups are called diols (using the IUPAC system) or glycols. Compounds with three hydroxy groups are called triols, and so forth. To name a diol, for example, the suffix -diol is added to the name of the parent alkane, and numbers are used in the prefix to indicate the loc ...
... Compounds with two hydroxy groups are called diols (using the IUPAC system) or glycols. Compounds with three hydroxy groups are called triols, and so forth. To name a diol, for example, the suffix -diol is added to the name of the parent alkane, and numbers are used in the prefix to indicate the loc ...
What is Organic Chemistry?
... if one electron is in the sigma and one in the sigma*, the molecule is of higher energy than the two separate atoms because the s* is slightly >∆E higher than the s orbitals while the s is ∆E lower Bonding orbital...high electron density between nuclei Antibonding orbital..node between nuclei (zero ...
... if one electron is in the sigma and one in the sigma*, the molecule is of higher energy than the two separate atoms because the s* is slightly >∆E higher than the s orbitals while the s is ∆E lower Bonding orbital...high electron density between nuclei Antibonding orbital..node between nuclei (zero ...
an introduction to organic reactions
... 1. Keep up with your studying day to day –– never let yourself get behind, or better yet, be a little ahead of your instructor. ...
... 1. Keep up with your studying day to day –– never let yourself get behind, or better yet, be a little ahead of your instructor. ...
Alkenes and Alkynes I
... Administration in 1992 for treatment of several types of cancer, including breast cancer, lung cancer, and melanoma An estimation: a 100-year old yew tree must be sacrificed in order to obtain 300 mg of Taxol, just enough for one single dose for a cancer patient Obviously, synthetic organic chemistr ...
... Administration in 1992 for treatment of several types of cancer, including breast cancer, lung cancer, and melanoma An estimation: a 100-year old yew tree must be sacrificed in order to obtain 300 mg of Taxol, just enough for one single dose for a cancer patient Obviously, synthetic organic chemistr ...
PowerPoint ******
... are almost ideally positioned to migrate because the bonds between the methyl groups and the rings are nearly coplanar with the empty orbitals of the cations. Equatorial substituents are unlikely to migrate. (4) Steric factors may also significantly affect the open-chain cations. Non-migrating subst ...
... are almost ideally positioned to migrate because the bonds between the methyl groups and the rings are nearly coplanar with the empty orbitals of the cations. Equatorial substituents are unlikely to migrate. (4) Steric factors may also significantly affect the open-chain cations. Non-migrating subst ...
New Stereoselective Approaches to Highly Substituted
... neutralised by the base and consequently cannot isomerise the initial products. Thus in theory, in the absence o f base, complete isomerisation of the initial products should occur and thus sulfonamide 34 was treated with iodine in acetonitrile to afford the 2,5-cis isomer 35b (Scheme 1.18; c). This ...
... neutralised by the base and consequently cannot isomerise the initial products. Thus in theory, in the absence o f base, complete isomerisation of the initial products should occur and thus sulfonamide 34 was treated with iodine in acetonitrile to afford the 2,5-cis isomer 35b (Scheme 1.18; c). This ...
Project Overview
... Professor William Tam received his B.Sc. at the University of Hong Kong in 1990 and his Ph.D. at the University of Toronto (Canada) in 1995. He was an NSERC postdoctoral fellow at the Imperial College (UK) and at Harvard University (USA). He joined the Department of Chemistry at the University of Gu ...
... Professor William Tam received his B.Sc. at the University of Hong Kong in 1990 and his Ph.D. at the University of Toronto (Canada) in 1995. He was an NSERC postdoctoral fellow at the Imperial College (UK) and at Harvard University (USA). He joined the Department of Chemistry at the University of Gu ...
Document
... • With base, the nucleophile is ¯OH, and the mechanism follows the usual two steps: nucleophilic attack followed by protonation. • The reaction rate increases in the presence of base because the base converts H2O into ¯OH, a stronger ...
... • With base, the nucleophile is ¯OH, and the mechanism follows the usual two steps: nucleophilic attack followed by protonation. • The reaction rate increases in the presence of base because the base converts H2O into ¯OH, a stronger ...
On The catalytic Hydrogenation of Co2 and Carboxylic acid esters
... equivalent is added to a substrate in the presence of a catalyst, catalytic hydrogenation reactions have the greatest impact on the human society. Discovered by Paul Sabatier in 1897,1 catalytic hydrogenation sparked the rapid development of the chemical industry. Probably the most remarkable exampl ...
... equivalent is added to a substrate in the presence of a catalyst, catalytic hydrogenation reactions have the greatest impact on the human society. Discovered by Paul Sabatier in 1897,1 catalytic hydrogenation sparked the rapid development of the chemical industry. Probably the most remarkable exampl ...
Diastereoselective Allylation of Carbonyl Compounds and Imines:
... the fact that in the synthesis of complex organic molecules, including natural products, the stereoselective allylations are more commonly performed with stoichiometric amounts of chiral reagents. In these reactions, the stereochemical information can be transferred by substrate diastereocontrol (su ...
... the fact that in the synthesis of complex organic molecules, including natural products, the stereoselective allylations are more commonly performed with stoichiometric amounts of chiral reagents. In these reactions, the stereochemical information can be transferred by substrate diastereocontrol (su ...
Reactions of Alkenes
... Two-step reaction sequence called hydroborationoxidation converts alkenes to alcohols with a regiochemistry opposite to Markovnikov's rule. ...
... Two-step reaction sequence called hydroborationoxidation converts alkenes to alcohols with a regiochemistry opposite to Markovnikov's rule. ...
Woodward–Hoffmann rules

The Woodward–Hoffmann rules, devised by Robert Burns Woodward and Roald Hoffmann, are a set of rules in organic chemistry predicting the barrier heights of pericyclic reactions based upon conservation of orbital symmetry. The Woodward–Hoffmann rules can be applied to understand electrocyclic reactions, cycloadditions (including cheletropic reactions), sigmatropic reactions, and group transfer reactions. Reactions are classified as allowed if the electronic barrier is low, and forbidden if the barrier is high. Forbidden reactions can still take place but require significantly more energy.The Woodward–Hoffmann rules were first formulated to explain the striking stereospecificity of electrocyclic reactions under thermal and photochemical control. Thermolysis of the substituted cyclobutene trans-1,2,3,4-tetramethylcyclobutene (1) gave only one diastereomer, the (E,E)-3,4-dimethyl-2,4-hexadiene (2) as shown below; the (Z,Z) and the (E,Z) diastereomers were not detected in the reaction. Similarly, thermolysis of cis-1,2,3,4-tetramethylcyclobutene (3) gave only the (E,Z) diastereomer (4).Due to their elegance and simplicity, the Woodward–Hoffmann rules are credited with first exemplifying the power of molecular orbital theory to experimental chemists. Hoffmann was awarded the 1981 Nobel Prize in Chemistry for this work, shared with Kenichi Fukui who developed a similar model using frontier molecular orbital (FMO) theory; because Woodward had died two years before, he was not eligible to win what would have been his second Nobel Prize for Chemistry.