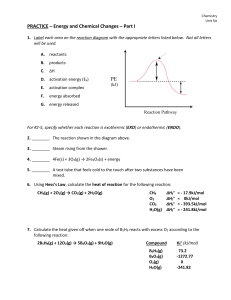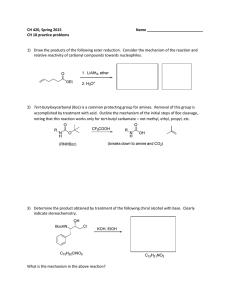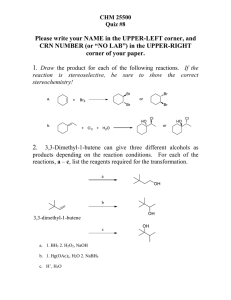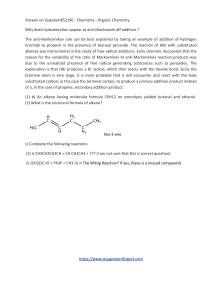
Answer on Question#52196 - Chemistry
... Answer on Question#52196 - Chemistry - Organic Chemistry Why does hydroboration appear as anti-Markownik off addition ? The anti-Markovnikov rule can be best explained by taking an example of addition of hydrogen bromide to propene in the presence of benzoyl peroxide. The reaction of HBr with substi ...
... Answer on Question#52196 - Chemistry - Organic Chemistry Why does hydroboration appear as anti-Markownik off addition ? The anti-Markovnikov rule can be best explained by taking an example of addition of hydrogen bromide to propene in the presence of benzoyl peroxide. The reaction of HBr with substi ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... Part-C Answer any four questions. Each question carries ten marks: ...
... Part-C Answer any four questions. Each question carries ten marks: ...
C1_5_products_from_oils_crossword
... 11. Polymers that change in response to changes in their environment. 12. A hydrocarbon whose molecules contain at least one carbon-carbon double bond. Down 1. Something that cannot be replaced once it is used up. 2. An alkene with the formula C2H4. 3. The reaction used in the oil industry to break ...
... 11. Polymers that change in response to changes in their environment. 12. A hydrocarbon whose molecules contain at least one carbon-carbon double bond. Down 1. Something that cannot be replaced once it is used up. 2. An alkene with the formula C2H4. 3. The reaction used in the oil industry to break ...
Midterm Exam 2
... molecule and then to solve the Schrödinger equation for the electrons at that nuclear separation. This can be illustrated with a molecular potential energy curve like the one shown below. In this diagram what does the bottom of the well represent? The point where the dotted lines intersect. ...
... molecule and then to solve the Schrödinger equation for the electrons at that nuclear separation. This can be illustrated with a molecular potential energy curve like the one shown below. In this diagram what does the bottom of the well represent? The point where the dotted lines intersect. ...
Syllabus
... Grading Policy: There will be 12 homework assignments, a midterm and a final. All are given on a take-home basis. If you have any question about a problem before starting to work, you are strongly encouraged to discuss the matter with the professor or fellow students. That is, students are encourage ...
... Grading Policy: There will be 12 homework assignments, a midterm and a final. All are given on a take-home basis. If you have any question about a problem before starting to work, you are strongly encouraged to discuss the matter with the professor or fellow students. That is, students are encourage ...
CHEM 201 Name Quiz 10 (Ch 17) ID Q1. Which of the following
... Q3. Which of the following reactions would not normally yield an alcohol? a) Oxymercuration/ demercuraction of ...
... Q3. Which of the following reactions would not normally yield an alcohol? a) Oxymercuration/ demercuraction of ...
國立屏東教育大學95學年度研究所碩士班入學考試
... 1. If matter is uniform throughout, cannot be separated into other substances by physical processes, but can be decomposed into other substances by chemical processes, it is called a (an) __________. (A) heterogeneous mixture (B) element (C) homogeneous mixture (D) compound (E) mixture of elements 2 ...
... 1. If matter is uniform throughout, cannot be separated into other substances by physical processes, but can be decomposed into other substances by chemical processes, it is called a (an) __________. (A) heterogeneous mixture (B) element (C) homogeneous mixture (D) compound (E) mixture of elements 2 ...
Assignment 2 Group A and B
... 9) Which of the following alcohols can be prepared by the reaction of methyl formate with excess Grignard reagent? A) 1-pentanol B) 2-pentanol C) 3-pentanol D) 2-methyl-2-pentanol E) 3-methyl-3-pentanol 10) What reagent(s) would you use to accomplish the following conversion? ...
... 9) Which of the following alcohols can be prepared by the reaction of methyl formate with excess Grignard reagent? A) 1-pentanol B) 2-pentanol C) 3-pentanol D) 2-methyl-2-pentanol E) 3-methyl-3-pentanol 10) What reagent(s) would you use to accomplish the following conversion? ...
Woodward–Hoffmann rules

The Woodward–Hoffmann rules, devised by Robert Burns Woodward and Roald Hoffmann, are a set of rules in organic chemistry predicting the barrier heights of pericyclic reactions based upon conservation of orbital symmetry. The Woodward–Hoffmann rules can be applied to understand electrocyclic reactions, cycloadditions (including cheletropic reactions), sigmatropic reactions, and group transfer reactions. Reactions are classified as allowed if the electronic barrier is low, and forbidden if the barrier is high. Forbidden reactions can still take place but require significantly more energy.The Woodward–Hoffmann rules were first formulated to explain the striking stereospecificity of electrocyclic reactions under thermal and photochemical control. Thermolysis of the substituted cyclobutene trans-1,2,3,4-tetramethylcyclobutene (1) gave only one diastereomer, the (E,E)-3,4-dimethyl-2,4-hexadiene (2) as shown below; the (Z,Z) and the (E,Z) diastereomers were not detected in the reaction. Similarly, thermolysis of cis-1,2,3,4-tetramethylcyclobutene (3) gave only the (E,Z) diastereomer (4).Due to their elegance and simplicity, the Woodward–Hoffmann rules are credited with first exemplifying the power of molecular orbital theory to experimental chemists. Hoffmann was awarded the 1981 Nobel Prize in Chemistry for this work, shared with Kenichi Fukui who developed a similar model using frontier molecular orbital (FMO) theory; because Woodward had died two years before, he was not eligible to win what would have been his second Nobel Prize for Chemistry.