Oxidation and Reduction - UCLA Chemistry and Biochemistry
... the number of bonds between a carbon and atoms that are more electronegative than the carbon (often oxygen). ...
... the number of bonds between a carbon and atoms that are more electronegative than the carbon (often oxygen). ...
CHMY_271_practice_exam_3
... 7. (8 pt) Give the major product for each of the following reactions, and explain why the products are not the same. H3O+ water O ...
... 7. (8 pt) Give the major product for each of the following reactions, and explain why the products are not the same. H3O+ water O ...
Combustion, Addition and Elimination Objective Combustion Example
... It may be possible to have more than one product from a reaction. Example: but-1-ene + hydrogen fluoride. ...
... It may be possible to have more than one product from a reaction. Example: but-1-ene + hydrogen fluoride. ...
CHEM 2411 – Organic Chemistry I Radicals/Radical Reactions 1
... 9) Draw the major product(s) of the following reaction. Is the product optically active? Explain. ...
... 9) Draw the major product(s) of the following reaction. Is the product optically active? Explain. ...
CHE 322
... conditions, to make the indicated large compound? In each case show the reaction that makes the C-C or C-O bond that links the pieces. All three must be different kinds of reactions. [Caution: parts of some reaction partners are missing in the given products due to replacement or subsequent reaction ...
... conditions, to make the indicated large compound? In each case show the reaction that makes the C-C or C-O bond that links the pieces. All three must be different kinds of reactions. [Caution: parts of some reaction partners are missing in the given products due to replacement or subsequent reaction ...
PowerPoint Presentation - No Slide Title
... Nucleophiles: electron-rich atoms or molecules that react with electrophiles. “electrophile”- likes electrons (likes minus: eand anions) Examples of nucleophiles ...
... Nucleophiles: electron-rich atoms or molecules that react with electrophiles. “electrophile”- likes electrons (likes minus: eand anions) Examples of nucleophiles ...
Snc2d Chapter 5 Practice Test
... d) Show a Bohr diagram above of P forming an ion, indicating beside your diagram the number of electrons gained or lost. Include the symbol with net charge and the name of the ion formed. e) With regard to ion formation how are metals different from nonmetals? (Two differences) ...
... d) Show a Bohr diagram above of P forming an ion, indicating beside your diagram the number of electrons gained or lost. Include the symbol with net charge and the name of the ion formed. e) With regard to ion formation how are metals different from nonmetals? (Two differences) ...
No Slide Title
... Nucleophiles: electron-rich atoms or molecules that react with electrophiles. “electrophile”- likes electrons (likes minus: eand anions) Examples of nucleophiles ...
... Nucleophiles: electron-rich atoms or molecules that react with electrophiles. “electrophile”- likes electrons (likes minus: eand anions) Examples of nucleophiles ...
Chapter One: Molecular Structure
... Bonding, Electronic Structural Models and Drawing Organic Molecules 1. Introduction to Organic Chemistry 2. What you need to remember from General Chemistry 3. Drawing Lewis Structures a. Octet vs non-octet structures b. e- book-keeping for octet structures: o #bonds = [Need-Have]/2 o Need = # neede ...
... Bonding, Electronic Structural Models and Drawing Organic Molecules 1. Introduction to Organic Chemistry 2. What you need to remember from General Chemistry 3. Drawing Lewis Structures a. Octet vs non-octet structures b. e- book-keeping for octet structures: o #bonds = [Need-Have]/2 o Need = # neede ...
N H CCl3 C O N CCl3 C Cl (ii) SOCl2 7.55 g 7.78 g CCl C N NH N H
... side chain? (Hint: a nitrogen atom which is part of a double bond, including aromatic N atoms such as in pyridine, are more basic than nitrogen atoms which have only single bonds to them.) (b) Write the neutral arginine molecule in its zwiterionic form. ...
... side chain? (Hint: a nitrogen atom which is part of a double bond, including aromatic N atoms such as in pyridine, are more basic than nitrogen atoms which have only single bonds to them.) (b) Write the neutral arginine molecule in its zwiterionic form. ...
Notes on Chapter 12 Chemical Equilibrium
... 2. Reaction rates = speed of the reaction = change in concentration/change in time which can be measured in terms of formation of product or loss of reactants over time. Factors that affect the rate of reaction: a. temperature- increase in temperature increases reaction rates b. concentration- incre ...
... 2. Reaction rates = speed of the reaction = change in concentration/change in time which can be measured in terms of formation of product or loss of reactants over time. Factors that affect the rate of reaction: a. temperature- increase in temperature increases reaction rates b. concentration- incre ...
the original file
... 1. how to draw resonance structures 2. meaning of conjugated vs isolated pi bonds 3. what an orbital is 4. be able to draw MO diagrams for allyl radical and cation and benzene, such as the one in Fig. 10.2 but you dont need to know how the MOs look, just the relative energy levels and how to put in ...
... 1. how to draw resonance structures 2. meaning of conjugated vs isolated pi bonds 3. what an orbital is 4. be able to draw MO diagrams for allyl radical and cation and benzene, such as the one in Fig. 10.2 but you dont need to know how the MOs look, just the relative energy levels and how to put in ...
Number of Electron Pairs Allowed Sigmatropic Rearrangement
... 1950 Nobel Prize in chemistry very useful, makes a six-membered ring with excellent control of stereochemistry ...
... 1950 Nobel Prize in chemistry very useful, makes a six-membered ring with excellent control of stereochemistry ...
الرقم الجامعي
... an atom. Use the examples of Li versus F atoms to show the relative differences in the 2s and 2p levels in these atoms. ------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------ ...
... an atom. Use the examples of Li versus F atoms to show the relative differences in the 2s and 2p levels in these atoms. ------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------ ...
A simple calorimeter was used as a vessel to measure the heat
... c) In the reaction above the initial temperature is 21.0˚C and the final temperature is 65.8˚C. Assume the density of KBr(aq) is 1.0g/mL. i. Calculate ΔT for this reaction ii. Clearly identify this reaction as exothermic or endothermic with the appropriate sign. ...
... c) In the reaction above the initial temperature is 21.0˚C and the final temperature is 65.8˚C. Assume the density of KBr(aq) is 1.0g/mL. i. Calculate ΔT for this reaction ii. Clearly identify this reaction as exothermic or endothermic with the appropriate sign. ...
Slides for Chapter 1-4 - Department of Chemistry and Physics
... Reaction is with inversion at reacting center Follows second order reaction kinetics Ingold nomenclature to describe characteristic step: ...
... Reaction is with inversion at reacting center Follows second order reaction kinetics Ingold nomenclature to describe characteristic step: ...
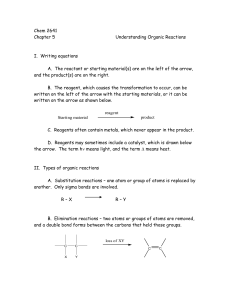
Chem 2641 Chapter 5 Understanding Organic Reactions I. Writing
... The reactant is converted directly to product. 2. Reactions that require more than one step contain species called intermediates. These species often have formal charges. C. Bonds can be broken homolytically or heterolytically. 1. Homolytic bond cleavage means that the two electrons of the bond divi ...
... The reactant is converted directly to product. 2. Reactions that require more than one step contain species called intermediates. These species often have formal charges. C. Bonds can be broken homolytically or heterolytically. 1. Homolytic bond cleavage means that the two electrons of the bond divi ...
Woodward–Hoffmann rules

The Woodward–Hoffmann rules, devised by Robert Burns Woodward and Roald Hoffmann, are a set of rules in organic chemistry predicting the barrier heights of pericyclic reactions based upon conservation of orbital symmetry. The Woodward–Hoffmann rules can be applied to understand electrocyclic reactions, cycloadditions (including cheletropic reactions), sigmatropic reactions, and group transfer reactions. Reactions are classified as allowed if the electronic barrier is low, and forbidden if the barrier is high. Forbidden reactions can still take place but require significantly more energy.The Woodward–Hoffmann rules were first formulated to explain the striking stereospecificity of electrocyclic reactions under thermal and photochemical control. Thermolysis of the substituted cyclobutene trans-1,2,3,4-tetramethylcyclobutene (1) gave only one diastereomer, the (E,E)-3,4-dimethyl-2,4-hexadiene (2) as shown below; the (Z,Z) and the (E,Z) diastereomers were not detected in the reaction. Similarly, thermolysis of cis-1,2,3,4-tetramethylcyclobutene (3) gave only the (E,Z) diastereomer (4).Due to their elegance and simplicity, the Woodward–Hoffmann rules are credited with first exemplifying the power of molecular orbital theory to experimental chemists. Hoffmann was awarded the 1981 Nobel Prize in Chemistry for this work, shared with Kenichi Fukui who developed a similar model using frontier molecular orbital (FMO) theory; because Woodward had died two years before, he was not eligible to win what would have been his second Nobel Prize for Chemistry.