Elimination Reactions
... regiochemistry/ Hofmann’s Rule • In bimolecular elimination reactions in the presence of either a bulky leaving group or a bulky base, the hydrogen that is lost will come from the LEAST highly-branched b-carbon. More branched ...
... regiochemistry/ Hofmann’s Rule • In bimolecular elimination reactions in the presence of either a bulky leaving group or a bulky base, the hydrogen that is lost will come from the LEAST highly-branched b-carbon. More branched ...
Document
... Alcohols, Ethers and Epoxides Reactions of Alcohols—Dehydration • The E1 dehydration of 20 and 30 alcohols with acid gives clean elimination products without any by-products formed from an SN1 reaction. • Clean elimination takes place because the reaction mixture contains no good nucleophile to rea ...
... Alcohols, Ethers and Epoxides Reactions of Alcohols—Dehydration • The E1 dehydration of 20 and 30 alcohols with acid gives clean elimination products without any by-products formed from an SN1 reaction. • Clean elimination takes place because the reaction mixture contains no good nucleophile to rea ...
Proofs to - Research Explorer
... counter-ion was inferred from microanalytical data for iodine and phosphorus (see experimental section) and was further confirmed by an X-ray structural investigation. Moreover, under similar reaction conditions (see below), the vinylidene complex 2 was also isolated as an iodide salt starting from ...
... counter-ion was inferred from microanalytical data for iodine and phosphorus (see experimental section) and was further confirmed by an X-ray structural investigation. Moreover, under similar reaction conditions (see below), the vinylidene complex 2 was also isolated as an iodide salt starting from ...
CH221 CLASS 13
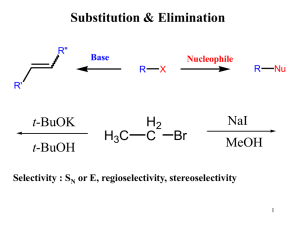
... Elimination is the opposite of addition and it has already been stated that alkenes can be produced by dehydrohalogenation of alkyl halides and by dehydration of alcohols. Although these are not necessarily the best routes to alkenes, two examples are considered here briefly, as a contrast to additi ...
... Elimination is the opposite of addition and it has already been stated that alkenes can be produced by dehydrohalogenation of alkyl halides and by dehydration of alcohols. Although these are not necessarily the best routes to alkenes, two examples are considered here briefly, as a contrast to additi ...
Elimination Reactions
... • E1 reactions do not show an isotope effect: kH/kD = 1 • This tells us that the C-D or C-H bonds are not broken in the rate determining step (step 1). They are broken in the fast step (step 2) in the mechanism). ...
... • E1 reactions do not show an isotope effect: kH/kD = 1 • This tells us that the C-D or C-H bonds are not broken in the rate determining step (step 1). They are broken in the fast step (step 2) in the mechanism). ...
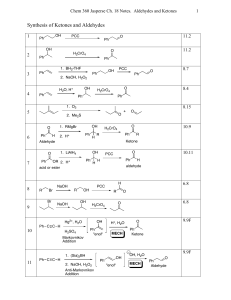
Synthesis of Ketones and Aldehydes
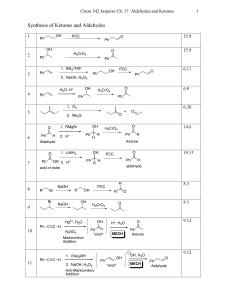
... Classification of Mechanisms Associated With Ketone/Aldehyde Reactions. • There may seem to be a dizzying number of mechanisms this chapter. But all of them simplify into some combination of acid- or base-catalyzed addition reaction, elimination reaction and/or substitution reaction. • To predict wh ...
... Classification of Mechanisms Associated With Ketone/Aldehyde Reactions. • There may seem to be a dizzying number of mechanisms this chapter. But all of them simplify into some combination of acid- or base-catalyzed addition reaction, elimination reaction and/or substitution reaction. • To predict wh ...
Modern Synthetic Methods for Copper-Mediated C(aryl
... Chan and co-workers[18] first described a collection of Nand O-arylation transformations under novel reaction conditions as early as in June 1997, but only part of the work was disclosed at this meeting. However in the first publication[14] it was already demonstrated that this method with a boronic ...
... Chan and co-workers[18] first described a collection of Nand O-arylation transformations under novel reaction conditions as early as in June 1997, but only part of the work was disclosed at this meeting. However in the first publication[14] it was already demonstrated that this method with a boronic ...
Catalytic Asymmetric Induction. Highly Enantioselective Addition of
... N M R spectra compared to liquid-state spectra makes the interpretation of the spectra cumbersome due to peak overlap. In some cases, such as porphyrins or phthalocyanines, these problems arising in the I3C spectra have been overcome by observing 15N instead of 13C.2-4 This requires considerable syn ...
... N M R spectra compared to liquid-state spectra makes the interpretation of the spectra cumbersome due to peak overlap. In some cases, such as porphyrins or phthalocyanines, these problems arising in the I3C spectra have been overcome by observing 15N instead of 13C.2-4 This requires considerable syn ...
Handout 3
... CH3 CH3 OH OH CH3 This is due to 1,2-Methyl shift that is similar to the hydride shift. In 1,2-Methyl shift a methyl group could move to an adjacent carbon atom to increase the stability of intermediate. CH3 CH3 H CH3C CHCH3 carbocation intermediate CH3CCH CH2 o + ve charge on (2 ) carbon atom CH3 C ...
... CH3 CH3 OH OH CH3 This is due to 1,2-Methyl shift that is similar to the hydride shift. In 1,2-Methyl shift a methyl group could move to an adjacent carbon atom to increase the stability of intermediate. CH3 CH3 H CH3C CHCH3 carbocation intermediate CH3CCH CH2 o + ve charge on (2 ) carbon atom CH3 C ...
Organic Chemistry Fifth Edition
... CH3Br + HO – CH3OH + Br – rate = k[CH3Br][HO – ] inference: rate-determining step is bimolecular ...
... CH3Br + HO – CH3OH + Br – rate = k[CH3Br][HO – ] inference: rate-determining step is bimolecular ...
The Grob Fragmentation
... - Generally if all the stereochemical requirements are met for a concerted mechanism (i.e. an anti-peri planar relationship) side reactions can be suppressed and you get a fast, high yielding reaction under simple base or acid catalysed conditions - It has also been used in more complex systems in o ...
... - Generally if all the stereochemical requirements are met for a concerted mechanism (i.e. an anti-peri planar relationship) side reactions can be suppressed and you get a fast, high yielding reaction under simple base or acid catalysed conditions - It has also been used in more complex systems in o ...
Chapter 4 Alcohols and Alkyl Halides
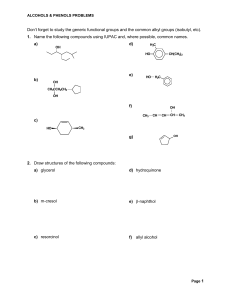
... Alcohols and Alkyl Halides Alcohols and alkyl halides are very important functional groups. A functional group is an atom or group of atoms that undergoes certain reactions that are typical of that functional group. It is important to recognize functional groups since it makes the organization and l ...
... Alcohols and Alkyl Halides Alcohols and alkyl halides are very important functional groups. A functional group is an atom or group of atoms that undergoes certain reactions that are typical of that functional group. It is important to recognize functional groups since it makes the organization and l ...
R - Evans - Harvard University
... reaction^.^^*^.^ In conjunction with our interests in amino acid derived natural products, we anticipated that these enolates might be attractive precursors to enantiomerically pure a-amino acids if effective electrophilic aminating agents could be developed. The simple strategy that was envisioned ...
... reaction^.^^*^.^ In conjunction with our interests in amino acid derived natural products, we anticipated that these enolates might be attractive precursors to enantiomerically pure a-amino acids if effective electrophilic aminating agents could be developed. The simple strategy that was envisioned ...
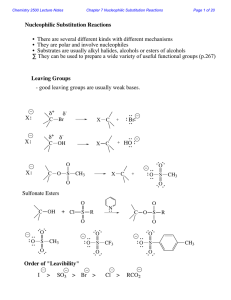
Nucleophilic Substitution Reactions
... The Hammond Postulate - widely used to rationalize differences in reaction rate "The structure of a transition state resembles that species to which it is closest in energy." Early T.S. ...
... The Hammond Postulate - widely used to rationalize differences in reaction rate "The structure of a transition state resembles that species to which it is closest in energy." Early T.S. ...
Woodward–Hoffmann rules

The Woodward–Hoffmann rules, devised by Robert Burns Woodward and Roald Hoffmann, are a set of rules in organic chemistry predicting the barrier heights of pericyclic reactions based upon conservation of orbital symmetry. The Woodward–Hoffmann rules can be applied to understand electrocyclic reactions, cycloadditions (including cheletropic reactions), sigmatropic reactions, and group transfer reactions. Reactions are classified as allowed if the electronic barrier is low, and forbidden if the barrier is high. Forbidden reactions can still take place but require significantly more energy.The Woodward–Hoffmann rules were first formulated to explain the striking stereospecificity of electrocyclic reactions under thermal and photochemical control. Thermolysis of the substituted cyclobutene trans-1,2,3,4-tetramethylcyclobutene (1) gave only one diastereomer, the (E,E)-3,4-dimethyl-2,4-hexadiene (2) as shown below; the (Z,Z) and the (E,Z) diastereomers were not detected in the reaction. Similarly, thermolysis of cis-1,2,3,4-tetramethylcyclobutene (3) gave only the (E,Z) diastereomer (4).Due to their elegance and simplicity, the Woodward–Hoffmann rules are credited with first exemplifying the power of molecular orbital theory to experimental chemists. Hoffmann was awarded the 1981 Nobel Prize in Chemistry for this work, shared with Kenichi Fukui who developed a similar model using frontier molecular orbital (FMO) theory; because Woodward had died two years before, he was not eligible to win what would have been his second Nobel Prize for Chemistry.