* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Handout 3
Woodward–Hoffmann rules wikipedia , lookup
Marcus theory wikipedia , lookup
Bottromycin wikipedia , lookup
Elias James Corey wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
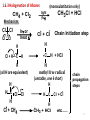
Physical organic chemistry wikipedia , lookup
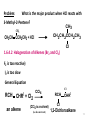
Petasis reaction wikipedia , lookup
Stille reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
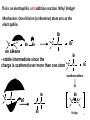
Asymmetric induction wikipedia , lookup
George S. Hammond wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
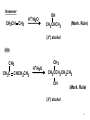
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
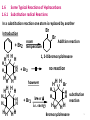
PHCM 331 – Organic and Medicinal/Pharmaceutical Chemistry I Handout # 3 Winter 2015 / 2016 The objectives of the 3rd handout are to know about: • Functional groups: Definition, Most common functional groups neutral, acidic, and basic functional groups, pKa values • Some Typical Reactions of Hydrocarbons • Radical Substitution Reactions, course, stability, and reactivity of radicals • Some free radical reaction mechanisms, Halogenation of alkanes, NBS alllylic substitution, • Free radical addition to alkenes (HBr / Peroxide addition), Radical polymerization of alkenes • Addition Reactions of Alkenes; Hydrohalogenation, Halogenation, Halohydrin Formation , Alcohols from, Syn addition of hydrogen • Addition to alkynes • Addition to dienes • Relative stabilities of conjugated and isolated dienes. 1 1.5 Functional groups 1.5.1 Definition It is the part of a molecule where most of its chemical reactions occur. Alkanes pKa 50 do not have a functional group. It is the part that effectively determines the compound’s chemical and most of its physical properties. 1.5.2 Most common functional groups; examples H Alkene R C C H Alcohol Amino acid pKa 25 R CH3OH Ether Alkyne pKa 40 pKa 16 R O R pKa alpha COOH 2.4 2 H O R C O R C O R Aldehyde Carboxylic ester R C O R Ketone O R C OH Carboxylic acid R NH2 Amine O R C NH2 Amide 3 3 Average values of pKa Alkanes 51 Amines 33-35 Esters 23-25 Ketones and aldehydes 18-20 Alcohols 15-19 b -Ketoesters 11 -Diketones 10 b Carboxylic acids 3-5 4 1.6 Some Typical Reactions of Hydrocarbons 1.6.1 Substitution radical Reactions In a substitution reaction one atom is replaced by another Br Introduction Br Addition reaction room + Br2 temperature H H HH H H HH H H H H HH HH H H HH H H HH 1, 2-Dibromocyclohexane no reaction + Br2 however + Br2 h or i.e. energy H H H H H Br HH H substitution H reaction HH Bromocyclohexane 5 Mechanism: How a given reaction takes place? In a chemical reaction some bonds are broken and others are formed via movement of electrons. In explaining a mechanism, movement of a pair of electrons is represented as follows: Movement of one electron as follows: RH an alkane Cl2 h RCl product R Intermediate (free radical) 1.6.2 course, stability, and reactivity of radicals stability of intermediate Why? o 3 > o o 2 > 1 > CH3 stability increases energy increases The energy needed to break C H bond depends on the type of C atom i.e. tert(3), sec-(2), primary-(1) or methyl. 6 The higher the amount of energy needed to break C H bond, the less stability of the intermediate (free radical) hence, the greater the tendency of the intermediate to recombine with hydrogen i.e. CH3 H CH3 + H Highly unstable More than 100 k cal/mol needed to break the C-H bond H H H H C C C H H H CH3 H 98 k cal/mol CH3 C H CH3 Bond dissociation energy = 91 k cal/mol only 77 7 1.6.3 Halogenation of Alkanes CH4 + Cl2 Mechanism: Cl Cl Cl hor heat Cl C CH3Cl + HCl light (h Chain Initiation step Cl + Cl H H Cl + H (monosubstitution only) H H (all H are equivalent) C H + HCl H methyl free radical (unstable, one ē short) H H C Cl H Cl + CH4 H Cl H C chain propagation steps Cl + Cl H CH3 + HCl etc..... 8 Cl2 Cl + Cl [Cl H3C + CH3 CH3CH3 CH3 + Cl CH3Cl Some examples 1) CH3CH3 + Cl2 2) CH3CH2CH3 Cl] [H3C CH3] Chain Termination Steps CH3CH2Cl Cl2/h CH3CHCH3 + CH3CH2CH2Cl Cl Major product Minor product The relative yield of products is dictated by the relative intermediates stabilities. in case 2 CH3CHCH3 is more stable than CH3CH2oCH2 CH3 (2o) (1 ) CH3 3)CH3 CH CH3 + Br2 h CH3 CH3 C Br CH3 CH3 CH3 CHCH2Br Minor product CH3 in case 3 CH3 C CH3 is more stable than CH3CHCH2 (3o) (1o) 9 1.6.4 Addition Reactions General Equation C C A A C B B product reagent An Alkene C The addition to alkenes is electrophilic. Mechanism. A B A+ + Belectrophile nucleophile A C C A+ C C carbocation (intermediate) A A B- C C C C B saturated compound 10 i.e. Addition to a double bond proceeds via two steps: 1An electrophile starts the reaction to produce an intermediate. 2- A nucleophile reacts with the intermediate to yield a neural molecule (product). 1.6.4.1 Hydrohalogenation of Alkenes X RCH CHR\ + HX an alkene e.g. CH2 RCH2CHR\ Hydrogen halide CH2 + HBr CH3CH2Br 11 However CH3CH CH2 + HBr CH2CHCH3 + CH3CH2CH2Br Br Mechanism: (Electrophilic addition; H+ then Br-) CH3CH CH2 + H+ CH3CHCH3 or CH3CH2 CH2 trace if any major more stable less stable (20) intermediate (10) intermediate Br Br CH3CHCH3 Br CH3CH2CH2 CH3CHCH3 Major product CH3CH2CH2Br Minor product 12 Problem: What is the major product when HCl reacts with 3-Methyl-2-Pentene? CH3 CH3 CH3CH2CCH2CH3 CH3CH CCH2CH3 + HCl Cl 1.6.4.2 Halogenation of Alkenes (Br2 and Cl2) F2 is too reactive) I2 is too slow General Equation RCH CHR\ + Cl2 an alkene CCl4 (CCl4 is a solvent) (i.e does not react) Cl RCH CHR\ Cl 1,2- Dichloroalkane 13 This is an electrophilic anti addition reaction. Why? Bridge! Mechanism: One chlorine (or Bromine) atom acts as the electrophile. Br C C Br C Br C Br an alkene Br - stable intermediate since the charge is scattered over more than one atom C C Br carbocation Br Br C C Br C Br C Br C C Bridge 14 example Br2 H organic solvent (absence of H2O) Br cyclopentene Br not H H H Br Br trans isomer cis isomer 1.6.4.3 Halohydrin Formation (Addition of X and OH) C Br C an alkene Br2/H2O C C i.e. anti addition OH Mechanism is similar to 1.7.2.2 however, water molecule is the nucleophile rather than bromide ion 15 Br C Br C Br - Br - C C H Br O C H C C OH Br Br2/CCl4 Br Br2/H2O CH3CHCHCH3 CH3CHCHCH3 OH 1.6.4.4 Alcohols from Alkenes Types of Alcohols CH3CH2OH H exists in higher concentration than Br - problems CH3CH CHCH3 -H + O H CH3CH CHCH3 C Br Br (Hydration i.e. addition of H2O) (1) alcohol [Ethyl alcohol; Ethanol] CH3CHCH3 OH CH3 CH3 C OH CH3 (2) alcohol [Isopropyl alcohol; Isopropanol] (3) alcohol [tert-Butyl alcohol; tert-Butanol] 16 Markovnikov’s Rule: on addition to double bonds, H is attached to the carbon with the most hydrogens. Negatively charged ion is considered a nucleophile. However, neutral H2O molecule (or ROH) may react as a nucleophile also using lone pair’s electrons of oxygen atom. Addition of X2 or X & OH: anti-addition (trans product). Acidic water addition of H, OH follows Mark.Rule (with possible rearrangement ).(hydride or methyl shift) Oxymercuration reaction is anti addition of H, OH that follows Mark.Rule (However, no rearrangement occurs). Hydroboration Method For the preparation of all types of alcohols 1°, 2°, 3° -Syn addition i.e. both H and OH are added to the same face of double bond. - Anti-Markovnikov’s addition -NO possible rearrangement. 17 17 A) The acidic water method H2O C C H /H2O No reaction because there is no electrophile. C C [H+ trace of inorganic acid e.g. HCl] H OH Mechanism: H CH2 CH2 H + (nucleophile) O (electrophile) CH3CH2 H ps. the electrophile may add to any of the two C atoms as both are equivalent CH3CH2 O H H -H+ (i.e. H+ is a catalyst) CH3CH2OH (1) alcohol since OH is attached to a (1) carbon atom. 18 However CH3CH CH2 H+/H2O OH CH3CHCH3 (Mark. Rule) (2°) alcohol also CH3 CH3C CHCH2CH3 H+/H2O CH3 CH3CCH2CH2CH3 OH (Mark. Rule) (3°) alcohol 19 Rearrangement Problem: In some cases, addition of water to alkenes is not a simple process. e.g. CH3 CH3 CH3 H / H2O CH3CCH CH2 CH3C CHCH3 minor CH3C CHCH3 major CH3 CH3 OH OH CH3 This is due to 1,2-Methyl shift that is similar to the hydride shift. In 1,2-Methyl shift a methyl group could move to an adjacent carbon atom to increase the stability of intermediate. CH3 CH3 H CH3C CHCH3 carbocation intermediate CH3CCH CH2 o + ve charge on (2 ) carbon atom CH3 CH3 To increase the stability of the intermediate, methyl group shifts as follows: CH3 1,2-methyl CH3C CHCH3 CH3 shift CH3 CH3C CHCH3 CH3 o more stable intermediate since +ve charge is located on (3 ) C atom This intermediate leads to the formation of the major product 20 B) Oxymercuration Method To avoid rearrangement problem, the following reagents are used instead of acidic water. Reagents 1) Mercuric acetate in water O O CH3C O Hg O CCH3 (abbreviated) AcO O Hg OAc Ac CH3C 2) Sodium Borohydride: NaBH4 (strong reducing reagent) 21 The reaction is anti addition of H, OH that follows Mark.Rule (no rearrangements). OH drives from water and H, (produced from NaBH4) replaces the electrophile HgOAc after it adds. Mechanism: C C AcO HgOAc HgOAc C C -OAc Stable intermediate, Hg helps stablizing ve charge The actual picture of the intermediate: as a result water Hg OAc C C attacks the opposite side i.e. (Anti) addition 22 HgOAc H O HgOAc C C H C H C HgOAc -H + C O H H OH C H+/H2O (rearrangement) CH3 CH3C CH CH3 CH2 NaBH4 C OH Conclusion: C CH3 CH3 C CH CH3 OH CH3 1) Hg(OAc)2/H2O 2) NaBH4 (no rearrangement) CH3 CH3C CHCH3 OH CH3 23 C) Hydroboration Method (Nobel Prize 1979) For the preparation of all types of alcohols 1°, 2°, 3°by choosing the right alkene -Syn addition i.e. both H and OH are added to the same face of the double bond. - Anti-Markovnikov’s addition due to what is called the steric effect. CH3CH2CH CH2 1) BH3/THF 2) H2O2 CH3CH2CH2CH2OH -NO possible rearrangement. Reagents THF 1) B2H6 (Diborane) (tetrahydrofuran) O 2) H2O2 (Hydrogen peroxide) H 2BH3 B OH H H Lewis acid "the electrophile" 24 Mechanism: The electrophile attacks the less substituted carbon atom. Why? The electrophile BH3 coordinates with the solvent THF and the actual attacking species is of a larger size: BH3 O e.g. O H B H H H H H C C H C H H BH3 H H H H B H C H C H3C X H B H CH3C C H H H H H2O2 HO B H H CH3CH2CH2OH 1 o alcohol 25 General Examples: CH3CH2CH 1) BH3/THF CH2 2) H2O2 CH3 CH3 1) BH3/THF CHCH3 2) H2O2 CH3C CH3CH2CH2CH2OH (1°) Alc. CH3CHCHCH3 (2°) Alc. OH CH3 CH3C C CH3 CH3 1) BH3/THF 2) H2O2 H3C OH CH3CH C CH3 (3°) Alc. CH3 26 (Review) OH CH3CH CH2 CH3 CH3C CHCH2CH3 H+/H2O (Mark. Rule) CH3CHCH3 (2°) alcohol CH3 H+/H2O CH3CCH2CH2CH3 (Mark. Rule) OH (3°) alcohol CH3CH CHCH3 Br Br2/CCl4 (CCl4 is a solvent) CH3CHCHCH3 (i.e does not react) CH3CH CHCH3 Br2/H2O Br Br CH3CHCHCH3 OH Intermediates may rearrange for more stability by moving a hydride or methyl group. The move should be 1,2 shift ONLY. CH3 CH3 1,2-methyl CH3C CHCH3 CH3C CHCH3 shift CH3 CH3 27 1.6.4.5 HBr/Peroxide/ h Free Radical Addition to Alkenes i.e. Addition of HBr in the presence of an organic peroxide ROOR. Don’t confuse organic peroxide e.g. CH3OOCH3 with inorganic peroxide e.g. HOOH (hydrogen peroxide). Recall: CH2 CH3CH CH3CHCH3 HBr Br electrophilic addition where H+ adds first followed by the bromide Br(i.e. Markovnikov's Rule) However in the presence of organic peroxide and light the addition is free radical . CH3CH . CH2 + HBr h RO OR Why? . In this case Br adds first instead of H e.g. CH3CH2CH2Br i.e. Anti Markovnikov's Rule 28 The mechanism h RO OR RO + H ROH + Br Br CH3CH CH2 +Br free radicals 2RO CH3CH (Step 1) CH2Br + CH2CHCH2 major (more stable) (Step 2) Br minor (less stable) CH3CHCH2Br + H Br CH3CH2CH2Br + Br (Step 3) Steps 2 and 3 are repeated over and over Conclusion: Markovnikov's Rule CH3CHCH3 HBr Br CH3CH CH2 HBr/Peroxide h CH3CH2CH2Br Anti Markovnikov's Rule 29 General Information: H2 gas is used for hydrogenation (reduction) however, under high pressure and the presence of a catalyst such as Pt, Pd, or Ni. Hydrogen-rich molecules are also used as a good source of hydrogen e.g. Sodium Borohydride: NaBH4 , LiAlH4 (strong reducing reagents) On the other hand, while O2 is used as oxidizing reagent, oxygen-rich molecules are also useful for the oxidation of organic molecules. e.g. O3 , H2O2 (hydrogen peroxide), and KMnO4 (potassium permanganate) Lone pair of electrons may be transferred into a single bond and vice versa i.e. a single bond may be transferred into lone pair. 30 1.6.4.6 Hydrogenation (reduction) of Alkenes addition of HH molecule (Syn) i.e. C C H H H2 is adsorbed on the surface of catalyst. catalyst RCH Catalyst CHR\ + H2 e.g. Pd, Ni or Pt RCH CHR\ H H syn addition CH3 CH3 CH3 H2/Pd H i.e. same side CH3 H 31 cis-1,2-Dimethylcyclopentane 1.6.5 Addition to Alkynes (H2, X2, HX and H2O) A) R Reduction of Alkynes H2/Catalyst C C R C C R C C H H cis isomer (less stable) syn addition R Li/NH3 (NH2Li + H) B) R Halogenation H R C R C H trans isomer (more stable) anti addition Br R R Br Br2/CCl4 Br 2 R C C another equiv.BrC (one equiv) R Br R trans-isomer anti addition (recall 1.6.4.2) CBr Br 32 C) Hydrohalogenation of Alkynes Br R C CH HBr RC Br CH2 HBr R C CH3 D) Addition of water to Alkynes : Tautomerism Br H O R C C R H2SO4/HgSO4 [H+/H2O] R C C R H RCH O C R\ H a ketone an enol i.e. the product is a mixture of the enol and keto forms. Tautomer: Isomeric equilibrium of 2 structures that differ in the arrangement of atoms. The acidic water method is used in making a ketone from an alkyne with the same # of C atoms. 33 1.6.6 Addition to Dienes (Electrophilic Addition) CH2 1 CH 2 CH 3 CH2 HCl one mole 4 [one equivalent] Why? Cl CH3CHCH CH2 + CH3 CH CHCH2Cl 1, 2-addition 1, 4-addition Mechanism (as in 1.7.2.1) CH2 CH CH CH2 H+ CH3 CH CH CH2 CH3 CH Cl 1, 2-addition CH CH2 Cl 1, 4-addition 34 1.6.6.1 Relative stabilities of conjugated and isolated dienes. There are three types of Dienes; conjugated, cumulated, & isolated. CH2 4 CH CH 3 2 s h orter th a n a s b o n d, h o wev er lo n g er th a n d. b. Why? CH2 1, 3-Butadiene (conjugated) 1 The most stable, lowest energy among other dienes. The p orbital pictures of dienes. H2C CH CH CH2 H2C CH CH CH2 most of the time i.e. sometimes H2C CH CH CH2 The Resonance Picture H2C CH CH CH2 CH2 i.e. the 2 central carbon atoms are linked together by a single bond that has some double bond character. CH CH CH2 sp3 no p orbitals CH2 5 CH CH2 4 3 CH CH2 1, 4-Pentadiene (isolated) 2 1 Less stable and of a higher energy than conjugated due to the absence of resonance. CH2 C CH2 1, 2-Propadiene (cumulated, allene) 3 2 1 Cumulated diene is the least stable (of highest energy) among other dienes. Why? Tutorial (molecular orbital picture)