technical bulletin reaction solvent dimethyl sulfoxide (dmso)
... DMSO is a versatile and powerful solvent that will dissolve most aromatic and unsaturated hydrocarbons, organic nitrogen compounds, organo-sulfur compounds and many inorganic salts. It is miscible with most of the common organic solvents such as alcohols, esters, ketones, lower ethers, chlorinated s ...
... DMSO is a versatile and powerful solvent that will dissolve most aromatic and unsaturated hydrocarbons, organic nitrogen compounds, organo-sulfur compounds and many inorganic salts. It is miscible with most of the common organic solvents such as alcohols, esters, ketones, lower ethers, chlorinated s ...
as a PDF
... It does not attempt to be a comprehensive treatise on the chemistry of these metals. It attempts to fill a slot between the general text and the in-depth review or monograph. The organometallic chemistry is confined to cr-bonded compounds in normal oxidation states; compounds with 7r-bonding ligands ...
... It does not attempt to be a comprehensive treatise on the chemistry of these metals. It attempts to fill a slot between the general text and the in-depth review or monograph. The organometallic chemistry is confined to cr-bonded compounds in normal oxidation states; compounds with 7r-bonding ligands ...
OC 2/e Ch 11
... • the OH group, however, is more acidic (pKa 16-18) than the terminal alkyne (pKa 25) • treating the compound with one mole of NaNH2 will give the alkoxide anion rather than the acetylide HC CCH2 CH2 CH 2 OH + N a+ NH 2 4-Pentyn-1-ol HC CCH2 CH2 CH 2 O- Na + + N H3 ...
... • the OH group, however, is more acidic (pKa 16-18) than the terminal alkyne (pKa 25) • treating the compound with one mole of NaNH2 will give the alkoxide anion rather than the acetylide HC CCH2 CH2 CH 2 OH + N a+ NH 2 4-Pentyn-1-ol HC CCH2 CH2 CH 2 O- Na + + N H3 ...
Alkenes 4 - ChemWeb (UCC)
... mercurinium or bromonium cation bond-breaking of the C-Hg or C-Br bond is more advanced than bond-formation with the incoming nucleophile. Hence the SN2 transition state for this reaction has partial carbocationic (i.e. SN1) character and therefore nucleophilic attack is at the most substituted carb ...
... mercurinium or bromonium cation bond-breaking of the C-Hg or C-Br bond is more advanced than bond-formation with the incoming nucleophile. Hence the SN2 transition state for this reaction has partial carbocationic (i.e. SN1) character and therefore nucleophilic attack is at the most substituted carb ...
Haloalkanes-haloarenes
... A. Rate of reaction depends on the slowest step, which involves the formation of carbocation. Greater the stability of the carbocation, greater will be its ease of formation from alkyl halide and faster the reaction. A tertiary carbocation is the most stable of the three, then comes 2o and least sta ...
... A. Rate of reaction depends on the slowest step, which involves the formation of carbocation. Greater the stability of the carbocation, greater will be its ease of formation from alkyl halide and faster the reaction. A tertiary carbocation is the most stable of the three, then comes 2o and least sta ...
Problems for Chapter 2
... PROB LE M 7 Four compounds having the formula C4H6O2 have the IR and NMR data given below. How many DBEs (double bond equivalents—see p. 75 in the textbook) are there in C4H6O2? What are the structures of the four compounds? You might again find it useful to draw a few structures to start with. (a) ...
... PROB LE M 7 Four compounds having the formula C4H6O2 have the IR and NMR data given below. How many DBEs (double bond equivalents—see p. 75 in the textbook) are there in C4H6O2? What are the structures of the four compounds? You might again find it useful to draw a few structures to start with. (a) ...
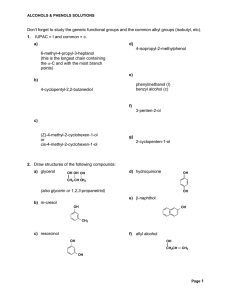
Don`t forget to study the generic functional groups and the common
... 18. Draw the structure(s) of all reagents which would give the following alcohol when reduced with LiAlH4 (CH3)2CHCH2OH ...
... 18. Draw the structure(s) of all reagents which would give the following alcohol when reduced with LiAlH4 (CH3)2CHCH2OH ...
Late Transition Metal Amido Complexes: Electronic
... about the M-N bonding, indicating a large polar -bond effect which results in a high anionic charge density on the nitrogen atom.[8] The negative charge can be effectively delocalized by NM -donation if vacant metal orbitals of suitable symmetry and energy are available, thus triggers a strengthe ...
... about the M-N bonding, indicating a large polar -bond effect which results in a high anionic charge density on the nitrogen atom.[8] The negative charge can be effectively delocalized by NM -donation if vacant metal orbitals of suitable symmetry and energy are available, thus triggers a strengthe ...
Alcohols, Ethers, and Epoxides
... The bond angle around the O atom in an alcohol or ether is similar to the tetrahedral bond angle of 109.5°. In contrast, the C – O – C bond angle of an epoxide must be 60°, a considerable deviation from the tetrahedral bond angle. For this reason, epoxides have angle strain, making them much more re ...
... The bond angle around the O atom in an alcohol or ether is similar to the tetrahedral bond angle of 109.5°. In contrast, the C – O – C bond angle of an epoxide must be 60°, a considerable deviation from the tetrahedral bond angle. For this reason, epoxides have angle strain, making them much more re ...
Preparation and reactions of some lower tungsten halides and
... oxide with carbon tetrachloride in a sealed tube at 280°C. (12) and the reduction of tungsten(VI) chloride with red phosphorus ...
... oxide with carbon tetrachloride in a sealed tube at 280°C. (12) and the reduction of tungsten(VI) chloride with red phosphorus ...
Lecture 8
... • Participation of acyl groups in on C2 promote 1,2-trans glycosidation. • Preferential formation of 1,2-cis product presents a greater challenge. – A glycosidation reaction employing an SN2 mechanism could be used to form the 1,2-cis product. – β-pyranosyl halides are too unstable for such an a ...
... • Participation of acyl groups in on C2 promote 1,2-trans glycosidation. • Preferential formation of 1,2-cis product presents a greater challenge. – A glycosidation reaction employing an SN2 mechanism could be used to form the 1,2-cis product. – β-pyranosyl halides are too unstable for such an a ...
Derivatization - Sigma
... Since the release of the last Derivatization guide in 2009, several innovative derivatization reagents have been introduced for various detection methods, and many other products and pack sizes have been modified or eliminated. This new version of the derivatization guide includes up-to-date informa ...
... Since the release of the last Derivatization guide in 2009, several innovative derivatization reagents have been introduced for various detection methods, and many other products and pack sizes have been modified or eliminated. This new version of the derivatization guide includes up-to-date informa ...
Chemical Redox Agents for Organometallic
... in the absence of resistance effects) make CPE ideal for forcing the uptake or release of one or more electrons from a substrate. The literature of CPE is well-developed for organic, inorganic, and organometallic systems.5,6 When CPE is coupled with voltammetric methods such as cyclic voltammetry, p ...
... in the absence of resistance effects) make CPE ideal for forcing the uptake or release of one or more electrons from a substrate. The literature of CPE is well-developed for organic, inorganic, and organometallic systems.5,6 When CPE is coupled with voltammetric methods such as cyclic voltammetry, p ...
Chemical Redox Agents for Organometallic
... in the absence of resistance effects) make CPE ideal for forcing the uptake or release of one or more electrons from a substrate. The literature of CPE is well-developed for organic, inorganic, and organometallic systems.5,6 When CPE is coupled with voltammetric methods such as cyclic voltammetry, p ...
... in the absence of resistance effects) make CPE ideal for forcing the uptake or release of one or more electrons from a substrate. The literature of CPE is well-developed for organic, inorganic, and organometallic systems.5,6 When CPE is coupled with voltammetric methods such as cyclic voltammetry, p ...
Silylation Overview - Sigma
... chapter. Now, it is easier to select the best protecting group for each functional group. The chapter “Silanisation” gives a short list of reagents, which can be used for surfaces like glass or silica gel, used for example in material sciences or in sealing techniques. Although dealing in principle ...
... chapter. Now, it is easier to select the best protecting group for each functional group. The chapter “Silanisation” gives a short list of reagents, which can be used for surfaces like glass or silica gel, used for example in material sciences or in sealing techniques. Although dealing in principle ...
Lecture Notes Chem 51B S. King Chapter 9 Alcohols, Ethers, and
... alcohols go undergo an E2 reaction. All three (1°, 2° and 3°) have the same first step, protonation of the hydroxyl group by the strong acid to make it a better LG: ...
... alcohols go undergo an E2 reaction. All three (1°, 2° and 3°) have the same first step, protonation of the hydroxyl group by the strong acid to make it a better LG: ...
Chiral Enolate Equivalents
... confines the range of usable electrophiles to aldehydes, some primary or activated alkyl halides, unsaturated carbonyls, electrophilic halogens, oxaziridines, aza compounds, and a handful of other reactive electrophiles.3 Intramolecular reactions may tolerate slightly less reactive electrophiles. Wi ...
... confines the range of usable electrophiles to aldehydes, some primary or activated alkyl halides, unsaturated carbonyls, electrophilic halogens, oxaziridines, aza compounds, and a handful of other reactive electrophiles.3 Intramolecular reactions may tolerate slightly less reactive electrophiles. Wi ...
Low Temperature Precursors for SnOx Thin Films
... Chapter 2-Heterocumulene insertions into tin(II) amide bonds Detailed descriptions of the synthesis, characterisation and precursor evaluation of tin(II) ureate compounds are presented in this chapter. The results of the precursor evaluation were used to select compound 4 for aerosol-assisted chemic ...
... Chapter 2-Heterocumulene insertions into tin(II) amide bonds Detailed descriptions of the synthesis, characterisation and precursor evaluation of tin(II) ureate compounds are presented in this chapter. The results of the precursor evaluation were used to select compound 4 for aerosol-assisted chemic ...
Excited State Reactions of Carbonyl Compounds
... The subtle dependence on the nature of the lowest excited state for a number of acetophenones, benzophenones and valerophenones on the substituent is listed in Table 9. 1. From the table it is clear that the triplet lifetime is very much dependent on the substituent on the aryl ring. For example, th ...
... The subtle dependence on the nature of the lowest excited state for a number of acetophenones, benzophenones and valerophenones on the substituent is listed in Table 9. 1. From the table it is clear that the triplet lifetime is very much dependent on the substituent on the aryl ring. For example, th ...
Derivatization reactions for the determination of amines by gas
... or nitrate [25]. Recent developments in environmental carcinogenesis have demonstrated that N-nitrosamines are potentially carcinogenic sub- ...
... or nitrate [25]. Recent developments in environmental carcinogenesis have demonstrated that N-nitrosamines are potentially carcinogenic sub- ...
Chapter 4 Metal nanoparticles stabilized by chiral ligands with carbohydrate backbone
... Recently, interest in chemical species of nanometric size has been growing.[1-17] Inorganic particles in solution, or colloids, as Graham described in 1861 as very slow sedimentation and noncrystalline state[18], have been known for ages. The first rational synthesis of gold colloids was described b ...
... Recently, interest in chemical species of nanometric size has been growing.[1-17] Inorganic particles in solution, or colloids, as Graham described in 1861 as very slow sedimentation and noncrystalline state[18], have been known for ages. The first rational synthesis of gold colloids was described b ...
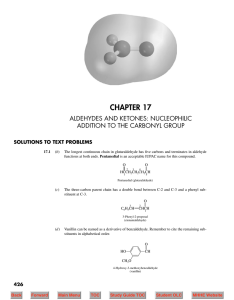
Nucleophilic Acyl Substitution
... Carboxylic acids containing six or fewer carbons are frequently called by their common names. These names were chosen by early chemists to describe some feature of the compound, usually its origin. For example, formic acid is found in ants, bees, and other stinging insects; its name comes from formi ...
... Carboxylic acids containing six or fewer carbons are frequently called by their common names. These names were chosen by early chemists to describe some feature of the compound, usually its origin. For example, formic acid is found in ants, bees, and other stinging insects; its name comes from formi ...
Organic Chemistry/Fourth Edition: e-Text
... Ethyl isopropyl ketone may be alternatively named 2-methyl-3-pentanone. Its longest continuous chain has five carbons. The carbonyl carbon is C-3 irrespective of the direction in which the chain is numbered, and so we choose the direction that gives the lower number to the position that bears the me ...
... Ethyl isopropyl ketone may be alternatively named 2-methyl-3-pentanone. Its longest continuous chain has five carbons. The carbonyl carbon is C-3 irrespective of the direction in which the chain is numbered, and so we choose the direction that gives the lower number to the position that bears the me ...
Lorell Thesis Final Version in PDF S
... overall. To my parents José and Betzaida, who always supported me in all aspects of life and made me what I am today. To my brother, José and sisters, Cheryl and Lorna for being there for me and giving me the unconditional love that only brothers can give. To my parents in law, Hilda and Luis, for a ...
... overall. To my parents José and Betzaida, who always supported me in all aspects of life and made me what I am today. To my brother, José and sisters, Cheryl and Lorna for being there for me and giving me the unconditional love that only brothers can give. To my parents in law, Hilda and Luis, for a ...
Stille reaction

The Stille reaction, or the Migita-Kosugi-Stille coupling, is a chemical reaction widely used in organic synthesis which involves the coupling of an organotin compound (also known as organostannanes) with a variety of organic electrophiles via palladium-catalyzed coupling reaction.The R1 group attached to the trialkyltin is normally sp2-hybridized, including alkenes, and aryl groups; however, conditions have been devised to incorporate both sp3-hybridized groups, such as allylic and benzylic substituents, and sp-hybridized alkynes. These organostannanes are also stable to both air and moisture, and many of these reagents are either commercially available or can be synthesized from literature precedent. However, these tin reagents tend to be highly toxic. X is typically a halide, such as Cl, Br, I, yet pseudohalides such as triflates and sulfonates and phosphates can also be used.The groundwork for the Stille reaction was laid by Colin Eaborn, Toshihiko Migita, and Masanori Kosugi in 1976 and 1977, who explored numerous palladium catalyzed couplings involving organotin reagents. John Stille and David Milstein developed a much milder and more broadly applicable procedure in 1978. Stille’s work on this area might have earned him a share of the 2010 Nobel Prize, which was awarded to Richard Heck, Ei-ichi Negishi, and Akira Suzuki for their work on the Heck, Negishi, and Suzuki coupling reactions. However, Stille died in the plane crash of United Airlines Flight 232 in 1989.Several reviews have been published on the Stille reaction.