* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 2.26 MB - KFUPM Resources v3
Thermal expansion wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Thermodynamics wikipedia , lookup
Work (thermodynamics) wikipedia , lookup
Supercritical fluid wikipedia , lookup
Surface tension wikipedia , lookup
Superfluid helium-4 wikipedia , lookup
Van der Waals equation wikipedia , lookup
Sessile drop technique wikipedia , lookup
Countercurrent exchange wikipedia , lookup
Ionic liquid wikipedia , lookup
Equation of state wikipedia , lookup
Glass transition wikipedia , lookup
Water vapor wikipedia , lookup
Liquid crystal wikipedia , lookup
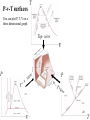
Ch.3 Properties of Pure Substances 1 Objectives Introduce the concept of a pure substance. Discuss the physics of phase-change processes. Illustrate the P-v, T-v, and P-T property diagrams and P-v-T surfaces of pure substances. Demonstrate the procedures for determining thermodynamic properties of pure substances from tables of property data. Describe the hypothetical substance “ideal gas” and the ideal-gas equation of state. Apply the ideal-gas equation of state in the solution of typical problems. Introduce the compressibility factor, which accounts for the deviation of real gases from ideal-gas behavior. Present some of the best-known equations of state. 2 Pure Substance In Chemistry you defined a pure substance as an Air element or a compound Something that can not be separated In Thermodynamics we’ll define it as something that has a fixed (same) chemical composition throughout Examples of Pure Substance Water, nitrogen, helium, and carbon dioxide, for example, are all pure substances. A mixture of water liquid and water vapor, for example, is a pure substance because both phases have the same chemical composition. N2 Water vapor Water liquid Pure substance 3 Example of Non-Pure Substance Air Gas A mixture of liquid air and gaseous air, however, is not a pure substance. Air liquid This is because different air components condense at different temperatures at a specified pressures and thus the composition of liquid air and gas air will be different.. Not a Pure substance Phases of Pure Substance A phase is identified as having a distinct molecular arrangement. This molecular arrangement is homogeneous throughout the system. The phase separated from the other phases by easily identifiable boundary surfaces. vapor liquid Solid 4 Solid Phase of Pure Substance The molecules in a solid are arranged in a lattice (network or pattern) that is repeated throughout. Three dimensional pattern Large attractive forces between atoms or molecules Molecule at relatively fixed position in solid. 5 Liquid Phase of Pure Substance When a solid reaches a sufficiently high temperature the velocity (and thus the momentum) of the molecules reach a point where the intermolecular forces are partially defeated and groups of the molecules break away (melting point) In liquid the molecular spacing is not much different from that of solids, except that they can rotate and translate freely (they are not at fixed positions relative to each other) Distance between molecules increase slightly as a solid turns to liquid 6 Gas Phase of Pure Substance In the gas phase, the molecules are far apart from each other, and a molecular order is nonexistent. Molecules move about at random, continually colliding with each other and the walls of the container they are in High kinetic energy In order to liquefy, lots of that kinetic energy must be released 7 Liquid phase to Gas Phase Let us study what would happen when we heat a liquid phase of pure substance at a constant pressure Liquid Water Pressure under the piston = Pressure above the piston Piston cylinder device – maintains constant pressure 8 T 5 2 3 Phase Change Processes on a Tv diagram 4 P is constant 1 Constant pressure line v 9 Phase Change Processes on a T-v diagram Consider a piston-cylinder device with water inside at 20oC and 1 atm pressure . At this P and T, water is called compressed (or subcooled) liquid state. Compressed liquid means that it is not about to vaporize. The system is heated and the piston is allowed to float and thus the pressure will be constant. T and v will increase until the system reaches 100 C at which any addition of heat will cause some of the liquid to vaporize The temperature at which a pure substance changes phase is called the saturation temperature, Tsat. At Tsat, Liquid and vapor phases are in equilibrium. A liquid that is about to vaporize is called Saturated Liquid. 10 Adding more heat will cause boiling to start. Liquid gradually evaporates (state 3) but temperature will remain constant, The only change is the increase in the specific volume (v) until it reaches state 4 (saturated vapor). Heating the system further, will increase both the temperature and specific volume (state 5). This single-phase state is called “Superheated vapor” Repeat this experiment for higher pressures. Similar curves will be obtained but at higher sat. temperature. Note that the sat. liquid specific volume (vsat,l ) will increase while the sat. vapor specific volume (vsat,g ) will decrease A substance between saturated liquid (state 2) and saturated vapor (state 4) is called saturated liquid-vapor mixture. A vapor that is about to condense is called Saturated vapor. 11 Latent Heat Def.: the amount of energy absorbed or released during a phase change process. Latent heat of fusion: the amount of energy absorbed during melting. Which is equal to the amount of energy released during freezing. Latent heat of vaporization : the amount of energy absorbed during vaporization which is equal to the amount of energy released during condensation. 12 Saturation Temperature and Pressure Water at a pressure of 101.325 kPa, Tsat is 100oC. Conversely, at a temperature of 100oC, Psat is 101.325 kPa. Latent heat: fusion and vaporization. The magnitude of the latent heats depend on the temperature or pressure at which the phase change occur. 13 Saturation Temperature and Pressure At a given pressure, the temperature at which a pure substance changes phase is called the saturation temperature. Likewise the pressure. 14 Critical Point The critical point is defined as the point at which the saturated liquid and saturated vapor states are identical. At the critical pressure, there will be no distinct phase change process. Instead, the specific volume of the substance will continually increase and at all times there will be only one phase present. The saturated liquid states can be connected by a line called the saturated liquid line. The saturated vapor states can be connected by another line, called the saturated vapor line, to form a phase dome. Three main regions can be identified. vsat,l and vsat,g will be the same and we speak of Pcrit, Tcrt, and vcrit. Critical properties in Table A-1 15 Phase Change Processes on a P-v diagram Decrease P gradually but keep T constant. State 1 T = 150 C Water boils at Psat The pressure at which a pure substance changes phase is called the saturation pressure Psat. At Psat, Liquid and vapor phases are in equilibrium. From State 2 to 4, no weights are removed (P=constant) and T is kept constant but heating causes liquid to vaporize. State 2 T = 150 C State 3 T = 150 C State 4 T = 150 C State 5 T = 150 C 1 2 3 4 5 16 P-v diagrams with Solid Phase P – v diagram of substance that contracts on freezing (most substance) P – v diagram of substance that expands on freezing (such as water) 17 Triple point Constant T Line Under some conditions all three phases of substance coexist in equilibrium at states along the triple line. The states on the triple line of substance have the same pressure and temperature but different v. The triple line appears as a point on the P-T diagram. The triple point of water occurs at T= 0.01 C and P=0.6113 Kpa vapor liquid Solid 18 Property Diagrams P-T diagram (or Phase diagram) The P-T diagram is often called phase diagram since all three phases are separated by three lines, namely the sublimation line (between solid and vapor regions), the vaporization line (between liquid and vapor regions), and the melting line (between solid and liquid). 19 T P-v-T surfaces You can plot P, T, V on a three dimensional graph Top view v e tur a r pe Tem P v P w e i v v P P Tv iew 20 T Thermodynamics Tables The relationship among thermodynamic properties are too complex to be expressed in simple equations and thus, properties are measured and/or calculated and then presented in a tabulated form. Table A-1 Saturated liquidvapor region Table A4 T entry Table A5 P entry w Su Tab at pe le er r (o hea A6 r v te ap d or ) two properties will fix the state. In two phase regions, any two properties (except P and T) will fix the state. P and T are dependent on each other. Table A7 Compresse Liquid In single-phase regions, any Table A8 Saturated ice-vapor 21 Enthalpy − A Combination Property In the analysis of certain types of processes, particularly in power generation and refrigeration, we frequently encounter the combination of internal energy U, and pressure-volume product PV. That is H U PV h u pυ Before 1930, h was referred to as heat content or total heat. After 1930, it is referred to as enthalpy (from the Greek word enthalpien which means to heat) 22 Saturated Liquid and Saturated Vapor States Table A-4 Saturated liquid-vapor mixture falls under the P-v (or T-v) dome. Its properties can be obtained from Water Tables A-4 and A-5 P = co ns t. T vf vg 23 Saturated Liquid and Saturated Vapor States Table A-5 In Table A-5 (page 832), Pressure is listed in the left column as the independent variable. Use whichever table is convenient. v fg vg v f h fg hg h f Enthalpy of vaporization or latent heat the amount of energy needed to vaporize a unite mass of saturated liquid at a given temperature or pressure 24 Example 3-1: Saturated Liquid and Saturated Vapor A rigid tank contains 50 kg of saturated liquid water at 90oC. Determine the pressure in the tank and the volume of the tank. (Table A-4) Always first you must know the state!!! (Answers: 70.14 kPa, 0.0518 m3) 25 Example 3-2: Saturated Liquid and Saturated Vapor A piston-cylinder device contains 0.06 m3 of saturated water vapor at 350- kPa pressure. Determine the temperature of the vapor and the mass of the vapor inside the cylinder. (Table A-5) (Answers: 138.86oC, 0.114 kg) 26 Example 3-3: Saturated Liquid and Saturated Vapor A mass of 200 g of saturated liquid water is completely vaporized at a constant pressure of 100 kPa. Determine (a) the volume change and (b) the amount of energy added to the water. (Answers: 0.3368 m3, 451.6 kJ) 27 Saturated Liquid-Vapor Mixture In the saturated liquid-vapor mixture, the mass fraction of vapor is called the QUALITY (x) and is expresses as m m x m m m g f g g total Derivation: V V f Vg mv m f v f mg v g ( m m g )v f m g v g v (1 x)v f xvg v v f x (v g v f ) v v f xv fg where v fg v g v f Gas mg vg P or T Liquid mf vf f f g g 28 Average Properties In the saturated mixture region, the average value of any intensive property y is given as: y y f x( yg y f ) y f x y fg where f stands for saturated liquid and g for saturated vapor. For example: v vf xvfg h hf xhfg u uf xufg s sf xsfg 29 Average Properties In the saturated mixture region, the average value of any intensive property y is given as: y y f x( yg y f ) 01 y f x y fg = yg where f stands for saturated liquid and g for saturated vapor. For example: When x = 0 we have all liquid, and y = yf When x = 1 we have all gas, and y = yf + yfg = yg The average properties of the mixtures are always between the values of the saturated liquid and the saturated vapor properties. That is y f yavg y g 30 X=0 X=1 31 Saturated Liquid-Vapor Mixture T Tsat at given P P= co ns t . T T Tsat P Psat at given T vf v vg v v f v v g at given P or T u f u u f at given P or T P Psat T= c P vg h f h h f at given P or T on st . v saturated mixture v Quality is an intensive property 32 Example 3- 4: Saturated Liquid-vapor mixture (continued) A rigid tank contains 10 kg of water at 90oC. If 8 kg of water is in the liquid form and the rest is in the vapor form, determine (a) the pressure in the tank and (b) the volume of the tank. (Answers: 70.14 kPa, 4.73 m3) 33 Example 3-5: Saturated Liquid-vapor mixture (continued) An 80-L vessel contains 4 kg of refrigerant 134a at a pressure of 160 kPa. Determine a) the temperature of the refrigerant, b) the quality, c) the enthalpy of the refrigerant, and d) the volume occupied by the vapor phase. (Answers: -15.62oC, 0.158, 62.7 kJ/kg, 0.0777 m3) 34 Superheated Vapor Table A-6 In the region to the right of the saturated vapor line, a substance exists as superheated vapor (single phase). P T= co ns t. vg v v 35 Superheated Vapor . T T Tsat at given P P= co ns t T Tsat P Psat at given T vf v vg v v v g at given P or T u u g at given P or T P Psat T= c P h h g at given P or T on st . superheated vapor vg v v 36 Example 3-6 Determine the internal energy of water at 200 kPa and 300°C. P= co ns t . T T Tsat vf vg v v Answer u= 2808.8 kJ/kg 37 Compressed liquid Table A-7 only for water In the region to the left of the saturated liquid line, a substance exists as compressed liquid. T v 38 Compressed Liquid P= co ns t . T T Tsat T Tsat at given P P Psat at given T vf vg v v v v f at given P or T u u f at given P or T h h f at given P or T compressed liquid 39 A general approximation In the absence of compressed liquid data (available only for water), a general approximation is to treat compressed liquid as saturated liquid at the given temperature. Such that Acceptable P Wrong P= 5 Mp T a. 5 Mpa T =2 264 80 T= 8 v vf Approximate value Precise value v y y 64 v vf Wrong value Precise value 0 v f @T If the compression is moderate, the properties do not vary significantly with pressure. But they do vary with temperature 40 Linear Interpolation A B 100 5 130 y 200 10 130 100 y 5 200 100 10 5 41 Linear Interpolation (Continued) Now X1= X = X2= T 140 143 145 Psat y1= 0.3615 y=? y2= 0.4154 y y1 x x1 y 2 y1 x 2 x1 x x1 y y1 ( y2 y1 ) x2 x1 Psat 0.3615 Psat 0.394 143 140 145 140 ( 0.4154 0.3615) kPa 42 Example 3-7 Superheated Vapor Determine the temperature of water at a state of P = 0.5 MPa and h = 2890 kJ/kg. First go to saturated liquid vapor table get hf and hg at 0.5 MPa T C 200 T 250 h kJ/kg 2855.8 2890 2961.0 (Answers: 216.4 oC) 43 Example on Compressed Liquid Example 3-8: Determine the internal energy of compressed liquid water at 80oC and 5 MPa using (a) data from the compressed liquid table and (b) saturated liquid data. What is the error involved in the second case? (Answers: 333.82 kJ/kg, 334.97 kJ/kg, 0.34%) pa 5M 263.99 80 80 44 Example 3-8 more 45 The Use of Steam Table to Determine Properties Example 3-9: Determine the missing properties and the phase descriptions in the following table for water. 46 Reference State and Reference Values The values of u, h, and s cannot be measured directly, and they are calculated from measurable properties using the relations between thermodynamic properties. However, those relations give the changes in properties, not the values of properties at specified state. For water, the state saturated liquid at 0.01oC is taken as the reference state, and the internal energy and entropy are assigned zero values at that sate. For refrigerant 134a, the state saturated liquid at -40oC is taken as the reference state, and the enthalpy and entropy are assigned zero values at that state. In thermodynamics we are concerned with the changes in properties, and the reference chosen has no consequences in the calculations. i.e. we always need the change in u, h, and s in the real life ….and not the absolute value even if the reference temp. is different we do not care….! 47