Climatology, Variability, and Trends in the US Vapor Pressure Deficit
... For any given RH, the VPD varies exponentially because of the Clausius–Clapeyron dependency of es (Ta ) on Ta . That is, at very low temperatures a given RH will correspond to a very small VPD while at high temperatures the same RH will correspond to a very high VPD. Similarly, a given VPD will corr ...
... For any given RH, the VPD varies exponentially because of the Clausius–Clapeyron dependency of es (Ta ) on Ta . That is, at very low temperatures a given RH will correspond to a very small VPD while at high temperatures the same RH will correspond to a very high VPD. Similarly, a given VPD will corr ...
Meteorology Practice Exam
... c. rainy night with light winds. d. clear night with light winds. ____ 41. When the air is saturated, which of the following statements is NOT correct? a. The air temperature equals the wet-bulb temperature. b. The relative humidity is 100 percent. c. The air temperature equals the dew point tempera ...
... c. rainy night with light winds. d. clear night with light winds. ____ 41. When the air is saturated, which of the following statements is NOT correct? a. The air temperature equals the wet-bulb temperature. b. The relative humidity is 100 percent. c. The air temperature equals the dew point tempera ...
Detailed modeling of the evaporation and thermal decomposition of
... completely remains in solution, therefore the possibility of an unlimited oversaturation is assumed. In the saturated case, a limited solubility is considered by precipitation of solid crystalline urea out of the liquid phase. In this case, the saturation pressure is described according to Raoult's ...
... completely remains in solution, therefore the possibility of an unlimited oversaturation is assumed. In the saturated case, a limited solubility is considered by precipitation of solid crystalline urea out of the liquid phase. In this case, the saturation pressure is described according to Raoult's ...
Modeling the Solubility of Nitrogen Dioxide in Water Using
... fugacity coefficients. Instead, K1 is often used to represent this system and estimated through eq 6, i.e., the partial pressures of NO2 and NO in the vapor phase, which may be derived from experimental measurements.4,5 Some models for K1 were proposed using empirical equations18,19 and nomograms.19,2 ...
... fugacity coefficients. Instead, K1 is often used to represent this system and estimated through eq 6, i.e., the partial pressures of NO2 and NO in the vapor phase, which may be derived from experimental measurements.4,5 Some models for K1 were proposed using empirical equations18,19 and nomograms.19,2 ...
Steam - Student Guide
... occurs in about 0.0002 seconds. When only slightly superheated (or with a quality of nearly 100 percent), steam enters a nozzle and the pressure drop causes the steam to reach a saturated condition, water droplets should appear immediately; however, the expansion takes place so rapidly that there is ...
... occurs in about 0.0002 seconds. When only slightly superheated (or with a quality of nearly 100 percent), steam enters a nozzle and the pressure drop causes the steam to reach a saturated condition, water droplets should appear immediately; however, the expansion takes place so rapidly that there is ...
Knowledge Check (Answer Key)
... occurs in about 0.0002 seconds. When only slightly superheated (or with a quality of nearly 100 percent), steam enters a nozzle and the pressure drop causes the steam to reach a saturated condition, water droplets should appear immediately; however, the expansion takes place so rapidly that there is ...
... occurs in about 0.0002 seconds. When only slightly superheated (or with a quality of nearly 100 percent), steam enters a nozzle and the pressure drop causes the steam to reach a saturated condition, water droplets should appear immediately; however, the expansion takes place so rapidly that there is ...
Steam - Nuclear Community
... A saturated vapor is a substance that exists as a pure vapor at saturation temperature and pressure. It is a substance in which any drop in temperature and/or rise in pressure will cause it to condense. The term "dry saturated vapor" indicates that the quality is 100 percent and the moisture content ...
... A saturated vapor is a substance that exists as a pure vapor at saturation temperature and pressure. It is a substance in which any drop in temperature and/or rise in pressure will cause it to condense. The term "dry saturated vapor" indicates that the quality is 100 percent and the moisture content ...
Steam - Nuclear Community
... A saturated vapor is a substance that exists as a pure vapor at saturation temperature and pressure. It is a substance in which any drop in temperature and/or rise in pressure will cause it to condense. The term "dry saturated vapor" indicates that the quality is 100 percent and the moisture content ...
... A saturated vapor is a substance that exists as a pure vapor at saturation temperature and pressure. It is a substance in which any drop in temperature and/or rise in pressure will cause it to condense. The term "dry saturated vapor" indicates that the quality is 100 percent and the moisture content ...
Mean evaporation and condensation coefficients based on energy
... As we proceed, it will be seen that the values of the coefficients and have a marked influence on the temperature jump at the interface. In particular they can be chosen such that the temperature jump is positive, while the classical models, which assume a constant coefficient (i.e., = 0), wil ...
... As we proceed, it will be seen that the values of the coefficients and have a marked influence on the temperature jump at the interface. In particular they can be chosen such that the temperature jump is positive, while the classical models, which assume a constant coefficient (i.e., = 0), wil ...
Nafion® Dryers and Humidifiers The Ten Most Common Questions
... Desiccant dryers function by binding water to an absorbant. The absorbant may be a solid (such as silica gel) or a liquid (such as sulfuric acid) that binds water to its chemical structure as water-ofhydration. Desiccants are very simple to understand and to operate. Unfortunately, like condensers, ...
... Desiccant dryers function by binding water to an absorbant. The absorbant may be a solid (such as silica gel) or a liquid (such as sulfuric acid) that binds water to its chemical structure as water-ofhydration. Desiccants are very simple to understand and to operate. Unfortunately, like condensers, ...
Chapter 5
... Yet sometimes we can see an increase in temperature with an increase in altitude, and this is called a temperature inversion. ...
... Yet sometimes we can see an increase in temperature with an increase in altitude, and this is called a temperature inversion. ...
Chapter 11 Absorption Air Conditioners
... needs to be relatively low to prevent crystallization. When solid lithium bromide salts form in a machine the operation stops. Although the salts can be dissolved with the addition of water, the machine is inoperative until the condition is remedied. Absorption machines need to be designed to operat ...
... needs to be relatively low to prevent crystallization. When solid lithium bromide salts form in a machine the operation stops. Although the salts can be dissolved with the addition of water, the machine is inoperative until the condition is remedied. Absorption machines need to be designed to operat ...
Pure Substances
... cylinder contains one kg of ice at –200C and one bar. When heat is transferred to the ice the pressure remains constant the specific volume increases slightly and the temperature increases until it reaches 0 0C, at which point the ice melts and temperature remains constant. This state is called satu ...
... cylinder contains one kg of ice at –200C and one bar. When heat is transferred to the ice the pressure remains constant the specific volume increases slightly and the temperature increases until it reaches 0 0C, at which point the ice melts and temperature remains constant. This state is called satu ...
AOS 330: Physics of the Atmosphere and Ocean I Class Notes
... We will be concerned mainly with (1) and (2). The constituents falling in categories (3) and (4) are often of great interest chemically, radiatively, or as pollutants, but these have a negligible effect on the bulk thermodynamic properties of air and will not be considered until later. Below about 10 ...
... We will be concerned mainly with (1) and (2). The constituents falling in categories (3) and (4) are often of great interest chemically, radiatively, or as pollutants, but these have a negligible effect on the bulk thermodynamic properties of air and will not be considered until later. Below about 10 ...
Water Vapor and Mechanical Work: A Comparison of
... Emanuel 1998; Emanuel 2003). It is argued here that, although many atmospheric phenomena can be viewed at least in part as heat engines, the analogy with a Carnot cycle often overestimates the conversion of internal energy into kinetic energy in the presence of water vapor. To better assess the role ...
... Emanuel 1998; Emanuel 2003). It is argued here that, although many atmospheric phenomena can be viewed at least in part as heat engines, the analogy with a Carnot cycle often overestimates the conversion of internal energy into kinetic energy in the presence of water vapor. To better assess the role ...
Chapter 12
... solution is one in which the dissolved and undissolved solute are in dynamic equilibrium with each other. A saturated solution represents the limit of a solute’s ability to dissolve in a given quantity of solvent. Example if we measure the concentration of glucose in its saturated solution at 20⁰C w ...
... solution is one in which the dissolved and undissolved solute are in dynamic equilibrium with each other. A saturated solution represents the limit of a solute’s ability to dissolve in a given quantity of solvent. Example if we measure the concentration of glucose in its saturated solution at 20⁰C w ...
June 2013
... would start losing chickens. Now, impacts the hay’s value as well. To view this graph, they have controllers in the chicken visit www.mesonet.org, and click on ‘Agriculture’ in (80°-84°F) where wind-chill cooling can keep birds comfortable. The houses, and they use a system the top menu. Then select ...
... would start losing chickens. Now, impacts the hay’s value as well. To view this graph, they have controllers in the chicken visit www.mesonet.org, and click on ‘Agriculture’ in (80°-84°F) where wind-chill cooling can keep birds comfortable. The houses, and they use a system the top menu. Then select ...
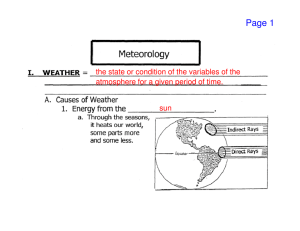
Meteorology
... determines that the body is too warm. b. Sweating increases in an effort to carry heat from deep inside the body to the surface of the skin. c. If water lost by sweating are not replaced, dehydration and heat exhaustion can result d. High humidity can interfere with the evaporation process that carr ...
... determines that the body is too warm. b. Sweating increases in an effort to carry heat from deep inside the body to the surface of the skin. c. If water lost by sweating are not replaced, dehydration and heat exhaustion can result d. High humidity can interfere with the evaporation process that carr ...
Closing the Global Water Vapor Budget with AIRS
... evaporation data from the Goddard Satellite-based Surface Turbulent Fluxes (GSSTF). The atmospheric total water vapor sink (S) is estimated from AIRS water vapor retrievals with MERRA winds (AIRS–MERRA S) as well as directly from the MERRA water vapor budget (MERRA–MERRA S). The global geographical ...
... evaporation data from the Goddard Satellite-based Surface Turbulent Fluxes (GSSTF). The atmospheric total water vapor sink (S) is estimated from AIRS water vapor retrievals with MERRA winds (AIRS–MERRA S) as well as directly from the MERRA water vapor budget (MERRA–MERRA S). The global geographical ...
Changes of State
... Explain the relationship between equilibrium and changes of state. Predict changes in equilibrium using Le Chatelier's Principle. Explain what is meant by equilibrium vapor pressure. Describe the processes of boiling, freezing, melting, and sublimation. Interpret phase diagrams. ...
... Explain the relationship between equilibrium and changes of state. Predict changes in equilibrium using Le Chatelier's Principle. Explain what is meant by equilibrium vapor pressure. Describe the processes of boiling, freezing, melting, and sublimation. Interpret phase diagrams. ...
1 SOLUTIONS
... · The Solubility of gases decreases with increasing temperature (the colder the water, the better the gases dissolve). Examples are: Ø soda water keeps its carbonation (CO2 gas) better at low temperatures (at room temperature they go “flat” faster) Ø cold oceans have a higher concentration ...
... · The Solubility of gases decreases with increasing temperature (the colder the water, the better the gases dissolve). Examples are: Ø soda water keeps its carbonation (CO2 gas) better at low temperatures (at room temperature they go “flat” faster) Ø cold oceans have a higher concentration ...
Chapter 3: Properties of Pure Substances
... liquid or gas (or vapor). Also these figures show that a substance may exist as a mixture of two phases during phase change, solid-vapor, solid-liquid, and liquidvapor. Water may exist in the compressed liquid region, a region where saturated liquid water and saturated water vapor are in equilibrium ...
... liquid or gas (or vapor). Also these figures show that a substance may exist as a mixture of two phases during phase change, solid-vapor, solid-liquid, and liquidvapor. Water may exist in the compressed liquid region, a region where saturated liquid water and saturated water vapor are in equilibrium ...
Acetic acid-water system thermodynamical correlation of vapor
... tetramer is not considered and isobaric data are directly handled : this is equivalent to neglecting the temperature effect on the activity coefficients. This is justified by the facts that the influence of both the tetramer formation and the temperature effect do not introduce serious error, on one ...
... tetramer is not considered and isobaric data are directly handled : this is equivalent to neglecting the temperature effect on the activity coefficients. This is justified by the facts that the influence of both the tetramer formation and the temperature effect do not introduce serious error, on one ...
5 Atmospheric Stability
... (WALR) (sometimes even called pseudo) adiabatic lapse rate. everything that we have learnt so far, it comes as now surprise that colder air contains less water vapour due to the adiabatic and gas laws, so that when a parcel of air reaches its saturation point (dewpoint), condensation begins and even ...
... (WALR) (sometimes even called pseudo) adiabatic lapse rate. everything that we have learnt so far, it comes as now surprise that colder air contains less water vapour due to the adiabatic and gas laws, so that when a parcel of air reaches its saturation point (dewpoint), condensation begins and even ...
Water vapor

Water vapor, or water vapour or aqueous vapor, is the gaseous phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Unlike other forms of water, water vapor is invisible. Under typical atmospheric conditions, water vapor is continuously generated by evaporation and removed by condensation. It is lighter than air and triggers convection currents that can lead to clouds.Water vapor is a relatively common atmospheric constituent, present even in the solar atmosphere as well as every planet in the Solar System and many astronomical objects including natural satellites, comets and even large asteroids. Likewise the detection of extrasolar water vapor would indicate a similar distribution in other planetary systems. Water vapor is significant in that it can be indirect evidence supporting the presence of extraterrestrial liquid water in the case of some planetary mass objects.Being a component of Earth's hydrosphere and hydrologic cycle, it is particularly abundant in Earth's atmosphere where it is also a potent greenhouse gas along with other gases such as carbon dioxide and methane. Use of water vapor, as steam, has been important to humans for cooking and as a major component in energy production and transport systems since the industrial revolution.