File
... Weather is the condition of the atmosphere at a certain time and place. What you may call weather is the water cycle at work. ...
... Weather is the condition of the atmosphere at a certain time and place. What you may call weather is the water cycle at work. ...
Properties of pure substance
... The temperature at which water starts boiling depends on the pressure; therefore, if the pressure is fixed, so is the boiling temperature. Water boils at 100°C at 1 atm pressure. Saturation temperature Tsat: is the temperature at which the liquid and vapour phases are in equilibrium at a given press ...
... The temperature at which water starts boiling depends on the pressure; therefore, if the pressure is fixed, so is the boiling temperature. Water boils at 100°C at 1 atm pressure. Saturation temperature Tsat: is the temperature at which the liquid and vapour phases are in equilibrium at a given press ...
Studying Topography, Orographic Rainfall, and Ecosystems
... much must the air cool for dew to form that night? Question 4: The relative humidity is 90% at a noon-time temperature of 75°F (297°K). At what temperature will dew form that night? Notice that dew will form at higher temperature if the relative humidity is greater. Question 5: If Td = T, what is re ...
... much must the air cool for dew to form that night? Question 4: The relative humidity is 90% at a noon-time temperature of 75°F (297°K). At what temperature will dew form that night? Notice that dew will form at higher temperature if the relative humidity is greater. Question 5: If Td = T, what is re ...
Theory
... In a cooling tower with open water circulation, heat is removed from water because of the material and heat exchange between the water and the ambient air. The cooling tower is a special form of heat exchanger because in addition to heat exchange, a material exchange also occurs as a result of evapo ...
... In a cooling tower with open water circulation, heat is removed from water because of the material and heat exchange between the water and the ambient air. The cooling tower is a special form of heat exchanger because in addition to heat exchange, a material exchange also occurs as a result of evapo ...
phase diagrams and IMF
... 1.) Explain why the formation of solid and/or liquid phase(s) would be favored under conditions of: Explain using the ideas of kinetic energy (molecular motion), how close together molecules are/could be, ability to be attracted to its neighbor: ...
... 1.) Explain why the formation of solid and/or liquid phase(s) would be favored under conditions of: Explain using the ideas of kinetic energy (molecular motion), how close together molecules are/could be, ability to be attracted to its neighbor: ...
chapter 3 - UniMAP Portal
... The temperature at which water starts boiling depends on the pressure; therefore, if the pressure is fixed, so is the boiling temperature. Water boils at 100C at 1 atm pressure. Saturation temperature Tsat: The temperature at which a pure substance changes phase at a given pressure. Saturation pres ...
... The temperature at which water starts boiling depends on the pressure; therefore, if the pressure is fixed, so is the boiling temperature. Water boils at 100C at 1 atm pressure. Saturation temperature Tsat: The temperature at which a pure substance changes phase at a given pressure. Saturation pres ...
Properties of Pure Substance
... The temperature at which water starts boiling depends on the pressure; therefore, if the pressure is fixed, so is the boiling temperature. Water boils at 100°C at 1 atm pressure. Saturation temperature Tsat: is the temperature at which the liquid and vapour phases are in equilibrium at a given press ...
... The temperature at which water starts boiling depends on the pressure; therefore, if the pressure is fixed, so is the boiling temperature. Water boils at 100°C at 1 atm pressure. Saturation temperature Tsat: is the temperature at which the liquid and vapour phases are in equilibrium at a given press ...
Do the Dewpoint! - UNI ScholarWorks
... On the activity website, click on the Sky, Temp, Dew Point, Weather map. Display or print this map for students to observe. Ask them what the symbols mean. What is something new that they see? Students should observe that there are two numbers on the left side of the station model circle. The upper ...
... On the activity website, click on the Sky, Temp, Dew Point, Weather map. Display or print this map for students to observe. Ask them what the symbols mean. What is something new that they see? Students should observe that there are two numbers on the left side of the station model circle. The upper ...
What is Weather.
... back by water vapor and carbon dioxide in the atmosphere. In a green house the glass roof acts like the carbon dioxide and water vapor in the air. It lets the light in to heat up the soil but does not let the infrared waves out. Gasses that thicken the atmosphere. – Water vapor ...
... back by water vapor and carbon dioxide in the atmosphere. In a green house the glass roof acts like the carbon dioxide and water vapor in the air. It lets the light in to heat up the soil but does not let the infrared waves out. Gasses that thicken the atmosphere. – Water vapor ...
Lab # 28: Calculating the Value of the Ideal Gas Constant “R”
... this lab, you will use the ideal gas law to calculate the value of the ideal gas constant “R”. We will collect a sample of gas from a lighter by water displacement. See Figure 1 for the set up. Here are a few “nuts and bolts” of the lab to keep in mind: - The volume of gas, V, is simply the volume o ...
... this lab, you will use the ideal gas law to calculate the value of the ideal gas constant “R”. We will collect a sample of gas from a lighter by water displacement. See Figure 1 for the set up. Here are a few “nuts and bolts” of the lab to keep in mind: - The volume of gas, V, is simply the volume o ...
Chapter 6 - Department of Chemical Engineering
... Vapor pressure of liquids depends on the temperature and the nature of the liquid. The forces causing the vaporization of a liquid are derived from the kinetic energy of translation of its molecules. An increase in kinetic energy of molecular translation increases the rate of vaporization and vapor ...
... Vapor pressure of liquids depends on the temperature and the nature of the liquid. The forces causing the vaporization of a liquid are derived from the kinetic energy of translation of its molecules. An increase in kinetic energy of molecular translation increases the rate of vaporization and vapor ...
Overview of the Earth`s Atmosphere
... • Check the website frequently for new announcements • “Refresh (Reload)” the page every time you visit, to make sure it displays the latest information ...
... • Check the website frequently for new announcements • “Refresh (Reload)” the page every time you visit, to make sure it displays the latest information ...
Met 61 - San Jose State University
... at constant pressure for the air to become saturated. Or, this is the temperature where the actual mixing ratio and the saturation mixing ratio are equal. Dewpoint is a good measure of the amount of water in the air. Td ~ 20°C uncomfortable Td > 24 °C very uncomfortable! The Clausius-Clapeyron can b ...
... at constant pressure for the air to become saturated. Or, this is the temperature where the actual mixing ratio and the saturation mixing ratio are equal. Dewpoint is a good measure of the amount of water in the air. Td ~ 20°C uncomfortable Td > 24 °C very uncomfortable! The Clausius-Clapeyron can b ...
Chapter 12: Intermolecular Attractions and the Properties of Liquids
... 1-Increasing temperature. It increases the amount of vapor and decreases the amount of liquid. At higher temperature, the total fraction of molecules with kinetic energy large enough to escape to vapor phase is larger so the rate of evaporation is larger. 2- Vapor pressure increases with decreasing ...
... 1-Increasing temperature. It increases the amount of vapor and decreases the amount of liquid. At higher temperature, the total fraction of molecules with kinetic energy large enough to escape to vapor phase is larger so the rate of evaporation is larger. 2- Vapor pressure increases with decreasing ...
Science Scientific Method - SOEST
... philosopher Aristotle was the first person to organize and record his weather thoughts in a systematic way. ~330 BC A student of Aristotle, Theophrates, wrote first book on weather forecasting. ~200 BC Hero developed crude water thermometer. ...
... philosopher Aristotle was the first person to organize and record his weather thoughts in a systematic way. ~330 BC A student of Aristotle, Theophrates, wrote first book on weather forecasting. ~200 BC Hero developed crude water thermometer. ...
Earth – The Water Planet
... Humidity is the amount of water vapor in the air. Relative humidity is defined as the ratio of the partial pressure of water vapor to the saturated vapor pressure of water vapor at a prescribed temperature. Humidity may also be expressed as specific humidity. Relative humidity is an important metric ...
... Humidity is the amount of water vapor in the air. Relative humidity is defined as the ratio of the partial pressure of water vapor to the saturated vapor pressure of water vapor at a prescribed temperature. Humidity may also be expressed as specific humidity. Relative humidity is an important metric ...
Classroom Teacher Preparation Earth Science 16: Weather
... Snow – water vapor frozen into ice crystals Sleet – ice pellets that form when raindrops hit a subfreezing patch of air near earth’s surface Hail – a mixture of liquid precipitation made up of layers of ice ...
... Snow – water vapor frozen into ice crystals Sleet – ice pellets that form when raindrops hit a subfreezing patch of air near earth’s surface Hail – a mixture of liquid precipitation made up of layers of ice ...
Q: What is Weather
... Closely spaced isobars - lines on a map that connect places of equal air pressure - indicate a steep pressure gradient and high winds Widely spaced isobars indicate a weak pressure gradient and light winds. Influence of temperature on weather Temperature: measure of the motion of the molecules in a ...
... Closely spaced isobars - lines on a map that connect places of equal air pressure - indicate a steep pressure gradient and high winds Widely spaced isobars indicate a weak pressure gradient and light winds. Influence of temperature on weather Temperature: measure of the motion of the molecules in a ...
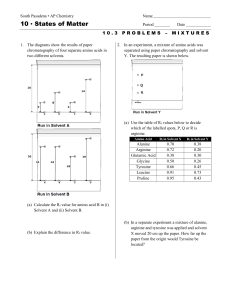
10 States of Matter
... 3. What is the vapor pressure of a solution by mixing 35.0 grams of urea (CH4N2O) with 150.0 grams of acetone (C3H6O) at 40°C? Assume urea is nonvolatile, and the vapor pressure of pure acetone is 400. torr at 40°C. ...
... 3. What is the vapor pressure of a solution by mixing 35.0 grams of urea (CH4N2O) with 150.0 grams of acetone (C3H6O) at 40°C? Assume urea is nonvolatile, and the vapor pressure of pure acetone is 400. torr at 40°C. ...
METEOROLOGY
... Inversion in the stratosphere is due to heating of stratosphere from the absorption of UV rays by O3; absence of O3 ---- air would become colder with height • Mesosphere: Extremely thin air, low pressure and density; average temp. ~-90°C; • Thermosphere: Hot layer above Mesosphere; very few atoms an ...
... Inversion in the stratosphere is due to heating of stratosphere from the absorption of UV rays by O3; absence of O3 ---- air would become colder with height • Mesosphere: Extremely thin air, low pressure and density; average temp. ~-90°C; • Thermosphere: Hot layer above Mesosphere; very few atoms an ...
Powerpoint
... • Inversion in the stratosphere is due to heating of stratosphere from the absorption of UV rays by O3; absence of O3 ---- air would become colder with height • Mesosphere: Extremely thin air, low pressure and density; average temp. ~-90°C; • Thermosphere: Hot layer above Mesosphere; very few atoms ...
... • Inversion in the stratosphere is due to heating of stratosphere from the absorption of UV rays by O3; absence of O3 ---- air would become colder with height • Mesosphere: Extremely thin air, low pressure and density; average temp. ~-90°C; • Thermosphere: Hot layer above Mesosphere; very few atoms ...
File
... 1. The average temperature, winds, and rainfall that a place experiences over a period of years make up its _____________. 2. The ____________________ is what happens on a particular day and this doesn’t always fit in with the overall climatic pattern. 3. The topics have weather that is much the sam ...
... 1. The average temperature, winds, and rainfall that a place experiences over a period of years make up its _____________. 2. The ____________________ is what happens on a particular day and this doesn’t always fit in with the overall climatic pattern. 3. The topics have weather that is much the sam ...
Water vapor

Water vapor, or water vapour or aqueous vapor, is the gaseous phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Unlike other forms of water, water vapor is invisible. Under typical atmospheric conditions, water vapor is continuously generated by evaporation and removed by condensation. It is lighter than air and triggers convection currents that can lead to clouds.Water vapor is a relatively common atmospheric constituent, present even in the solar atmosphere as well as every planet in the Solar System and many astronomical objects including natural satellites, comets and even large asteroids. Likewise the detection of extrasolar water vapor would indicate a similar distribution in other planetary systems. Water vapor is significant in that it can be indirect evidence supporting the presence of extraterrestrial liquid water in the case of some planetary mass objects.Being a component of Earth's hydrosphere and hydrologic cycle, it is particularly abundant in Earth's atmosphere where it is also a potent greenhouse gas along with other gases such as carbon dioxide and methane. Use of water vapor, as steam, has been important to humans for cooking and as a major component in energy production and transport systems since the industrial revolution.