* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Studying Topography, Orographic Rainfall, and Ecosystems
Hyperthermia wikipedia , lookup
Atmospheric circulation wikipedia , lookup
Indoor air quality wikipedia , lookup
Water vapor wikipedia , lookup
Cold-air damming wikipedia , lookup
Satellite temperature measurements wikipedia , lookup
Air quality law wikipedia , lookup
Atmosphere of Earth wikipedia , lookup
Instrumental temperature record wikipedia , lookup
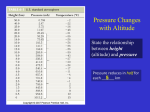
Draft Basic Lesson 1 StudyingTopography,OrographicRainfall,andEcosystems(STORE) BasicLesson1:PrinciplesofMeteorology Introduction This lesson defines several meteorological principles that are used throughout the STORE curriculum. Understanding of these principles is fundamental to the STORE curriculum. Objective To teach students fundamental meteorological concepts and their mathematical expression. LessonDuration 1 class period, 45 to 55 minutes Requirements Scientific calculator Part1‐RelativeHumidity Like all gases, air exerts pressure outwards against the boundaries containing it. The amount of pressure exerted is proportional to the density of the air, which varies with the moisture content. Dry air is composed of 78% nitrogen and 21% oxygen, which have molecular weights of 28 and 32, respectively. Water vapor has a molecular weight of 18 (i.e., lower than the predominant components of air). Because water vapor has a lower molecular weight than dry air, adding water vapor to dry air reduces its density. Moist air is less dense than dry air at the same temperature. If dry air is in contact with a body of water, water will evaporate and enter the air. This process will continue until the air is saturated and cannot hold any more water vapor. At saturation, the amount of water condensing and evaporating are equal and the amount of water vapor in the air does not change. Figure 1. State of Equilibrium. .(Source: University of Illinois) 1 Draft Basic Lesson 1 Relative humidity indicates the degree of moisture in the air. At 100% relative humidity, the air is saturated. The amount of water vapor is measured by the partial pressure (Pv) in units of millibars. Relative humidity is the amount of moisture in the air (vapor pressure or Pv) / maximum possible amount of moisture at saturation (vapor pressure in a state of saturation or Ps). Mathematically, relative humidity (r) is expressed as a percentage calculated using equation 1: Eq. 1 r = 100 Pv/Ps Where Pv and Ps are expressed in millibars. The amount of water vapor in the air at saturation depends on the temperature. Warm air can hold much more water vapor than cold air. Figure 2, below, shows the water vapor content for saturated air at various temperatures. Notice that the ability of air to hold water vapor increases greatly at higher air temperatures. Saturated Water Content (g/m3 ) 60.0 50.0 40.0 30.0 20.0 10.0 0.0 ‐10 0 10 20 30 40 Ambient Air Temperature (°C) Figure 2. Saturated water content versus ambient air temperature. Question 1: What is the relationship between air temperature, saturated H20 content, and relative humidity? Provide a short answer. Hint*: look at the equation for relative humidity and Figure 2. 2 Draft Basic Lesson 1 Part2‐RelationshipbetweenPressure,ElevationandTemperature As air (or any other gas) expands, its temperature drops. Conversely, when air is compressed, its temperature rises. Air pressure typically decreases with elevation above the earth’s surface because the column of air pressing down is less at higher elevations. Lower pressure allows air to expand, causing its temperature to decrease. Question 2: What is the relationship between elevation, pressure and temperature? Provide a short answer. Part3‐TemperatureLapseRate The troposphere (the lowest layer of the atmosphere) ranges from the earth’s surface up to about 10,000 meters (m) above sea level. Within the troposphere, temperature normally decreases with increasing elevation. In some circumstances, cold air is trapped near the ground and temperature increases with elevation, this is called an inversion. We assume the normal situation of decreasing air temperature with elevation (i.e., no inversion). The adiabatic temperature lapse rate is the rate at which a parcel of air cools as it rises upwards above sea level (adiabatic means without the addition or loss of heat). Eq. 2 lapse rate = dT/dz where dT is the change in temperature and dz is the change in elevation The air may rise because the air temperature is warmer near the ground surface than at elevation (warm air rises relative to colder air) and/or orographic (i.e., mountain) effects. Air encountering mountains will be pushed up and over the mountains by the prevailing winds. The temperature lapse rate of the atmosphere is measured by the National Weather Service (NWS) using meteorological balloons that are released twice daily at over 100 locations across the U.S. The balloons record temperature, pressure and humidity at intervals as they rise through the atmosphere and radio the data back to ground stations called “Upper Air Facilities”. Figure 3 (a and b) shows data collected by an NWS balloon released from Edwards Air Force Base (AFB), CA, on August 4, 2010. Figure 3a shows this data converted to western units. Figure 3b shows this data in metric units (as this data was collected in metric units). 3 Draft Basic Lesson 1 Figure 3a. Integrated Global Radiosonde Balloon Release, Edwards AFB, CA (August 4, 2010) [Western Units]. Figure 3b. Integrated Global Radiosonde Balloon Release, Edwards AFB, CA (August 4, 2010) [Metric Units]. 4 Draft Basic Lesson 1 The balloon release point is at an elevation of 724 m above mean sea level (MSL). Between 777 m and 9,690 m MSL, the air temperature decreases by about 57.3°C (see Figure 4). The temperature lapse rate is: 57.3°C / 8,913 m = 6.4°C / 1,000 m Part4‐DewPoint As we saw in Figure 2, lower temperature is associated with lower Ps. If the amount of water vapor in the air remains constant, the relative humidity (r) will increase as the air cools. If the air cools sufficiently, the amount of moisture in the air (i.e., Pv) will equal Ps. At this point, r = 1 and the air is saturated. The temperature at this point is called the Dew point. Dew point = temperature at which air becomes saturated. Additional cooling will result in condensation which removes water vapor from the air. Equation 3 allows calculation of dew point based on the relative humidity (r) and the air temperature (T). Eq. 3 Td = (r/100)1/8 (112+0.9T) + 0.1T – 112 where Td = dew point temperature in °Kelvin (K) r = relative humidity, and T = is the air temperature in °K Note: 273.15°K = 0°C = 32°F. Question 3: The relative humidity is 70% at a noon-time temperature of 75°F (297°K). How much must the air cool for dew to form that night? Question 4: The relative humidity is 90% at a noon-time temperature of 75°F (297°K). At what temperature will dew form that night? Notice that dew will form at higher temperature if the relative humidity is greater. Question 5: If Td = T, what is relative humidity? Question 6: As shown in Figure 3, the NWS meteorological balloon released from Edwards AFB , CA on August 4, 2010 measured a temperature lapse rate from the surface to an elevation of 9,000 m of 6.1°C/1,000 m. During initial release of the balloon, the temperature and relative humidity at the ground surface (i.e., sea level) was recorded as 18.8°C and 70.4% respectively. At what elevation will the air become saturated? Hint*: first calculate the dew point. The unsaturated air will rise until it reaches the elevation where it becomes saturated (e.g., 2,515 m). Further cooling by rising will result in condensation. During condensation, latent heat is released (the opposite of the latent heat absorbed during evaporation) causing the air to warm. As 5 Draft Basic Lesson 1 a result the temperature lapse rate decreases to what is called the saturated lapse rate. Therefore, the saturated (e.g., moist) lapse rate is less than the dry lapse rate. Another interesting point is that water vapor is less dense than air at the same temperature and pressure. Since condensation removes water vapor from the air, it also increases the density of the air. The denser drier air tends to sink within the atmosphere, resulting in instability and turbulence within the atmosphere. Sometimes a thin layer cold air is trapped near the ground surface and warm air occurs above it. This is called an inversion: the air temperature increases with increasing elevation until the inversion elevation is reached. Above the inversion level, the normal temperature lapse occurs. Ground fog is indicative of an inversion. The relatively cool air below the inversion level is saturated and forms fog and the warmer air above the inversion is not saturated. The result is fog that stays near the ground. 6 Draft Basic Lesson 1 Part5‐Precipitation Figure 4. Orographic lifting of clouds over steep-sided mountains. (Source: Rogers, David J., and Karl Hasselmann, Missouri University of Science and Technology) Precipitation occurs when water vapor in the atmosphere condenses (or freezes) and falls to earth. For this to occur, the condensed water must be in large enough droplets to be pulled to the ground surface by gravity (overcoming rising air or updrafts that tend to keep the drop aloft). Additionally, the water (or ice) droplet will encounter warmer air as it falls to earth and may evaporate before it reaches the ground surface. In addition to forming when the air is saturated (i.e., relative humidity = 100%), water droplets can also occur when the relative humidity is less than 100% due to condensation of water vapor on dust particles in the atmosphere. Question 7: Why is relative humidity important? Part6–Weathervs.Climate Temperature, precipitation and humidity, wind speed, cloud cover, and other physical conditions in the lower atmosphere over the short-term (e.g., hours or days) is referred to as weather and patterns of these variables over the long-term (e.g., decades to thousands of years) is climate. ClassLaboratory/FieldDemonstrations: Relative humidity: http://www.srh.noaa.gov/jetstream/atmos/ll_rainman.htm Air pressure: http://www.srh.noaa.gov/jetstream/atmos/ll_crunch.htm Dew point: http://www.crh.noaa.gov/abr/?n=humidity.php 7 Draft Basic Lesson 1 AdditionalInformation http://eo.ucar.edu/webweather/index.html hands-on science activities: http://eo.ucar.edu/webweather/activities.html “Layers of the Atmosphere” at http://www.srh.noaa.gov/jetstream/atmos/layers.htm Bibliography Bras, Rafael L., Hydrology: an introduction to hydrologic science (1990). Ritter, Michael E., The Physical Environment: an Introduction to Physical Geography (2006). 8