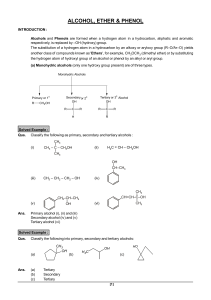
technical bulletin reaction solvent dimethyl sulfoxide (dmso)
... Chemically, DMSO is stable above 100° C in alkaline, acidic or neutral conditions. Prolonged refluxing at atmospheric pressure will cause slow decomposition of DMSO. If this occurs, it can be readily detected by the odor of trace amounts of methyl mercaptan and bis(methylthio)methane. The rate of de ...
... Chemically, DMSO is stable above 100° C in alkaline, acidic or neutral conditions. Prolonged refluxing at atmospheric pressure will cause slow decomposition of DMSO. If this occurs, it can be readily detected by the odor of trace amounts of methyl mercaptan and bis(methylthio)methane. The rate of de ...
Chapter 16 Aldehydes and Ketones
... An aldehyde cannot have the molecular formula C5H12O. C5H12 has too many H’s. Since an aldehyde has a double bond, the number of C’s and H’s resembles an alkene, not an alkane. An aldehyde with 5 C’s would have the molecular formula C5H10O. ...
... An aldehyde cannot have the molecular formula C5H12O. C5H12 has too many H’s. Since an aldehyde has a double bond, the number of C’s and H’s resembles an alkene, not an alkane. An aldehyde with 5 C’s would have the molecular formula C5H10O. ...
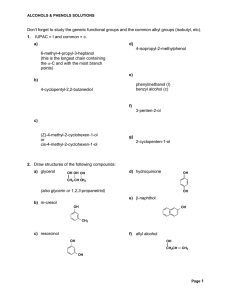
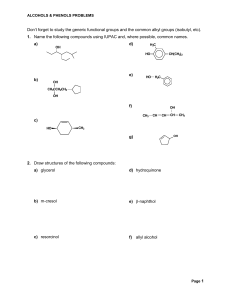
Don`t forget to study the generic functional groups and the common
... K (like Na and Li) is a very strong base and deprotonates the weakly acidic alcohol in an acid/base reaction. This is a rapid lab test for identifying alcohols; H2 gas is evolved. ...
... K (like Na and Li) is a very strong base and deprotonates the weakly acidic alcohol in an acid/base reaction. This is a rapid lab test for identifying alcohols; H2 gas is evolved. ...
Alkenes 4 - ChemWeb (UCC)
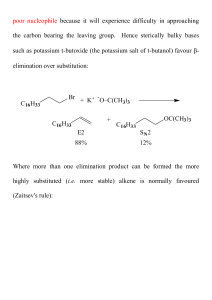
... Unlike the case of the E2 mechanism - there is no steric requirement for the conformation of the substrate in an E1 reaction. ...
... Unlike the case of the E2 mechanism - there is no steric requirement for the conformation of the substrate in an E1 reaction. ...
OC 2/e 9 Alcohols
... chromic acid oxidation of a CHO group, it is the hydrated form that is oxidized O R- C-H ...
... chromic acid oxidation of a CHO group, it is the hydrated form that is oxidized O R- C-H ...
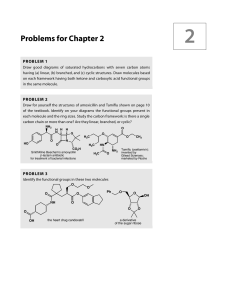
Problems for Chapter 2
... PROB LE M 7 Four compounds having the formula C4H6O2 have the IR and NMR data given below. How many DBEs (double bond equivalents—see p. 75 in the textbook) are there in C4H6O2? What are the structures of the four compounds? You might again find it useful to draw a few structures to start with. (a) ...
... PROB LE M 7 Four compounds having the formula C4H6O2 have the IR and NMR data given below. How many DBEs (double bond equivalents—see p. 75 in the textbook) are there in C4H6O2? What are the structures of the four compounds? You might again find it useful to draw a few structures to start with. (a) ...
OC 2/e Ch 11
... 11 Ethers - Protecting Grps • the new C-C bond can be formed by alkylation of the acetylide anion • the OH group, however, is more acidic (pKa 16-18) than the terminal alkyne (pKa 25) • treating the compound with one mole of NaNH2 will give the alkoxide anion rather than the acetylide HC CCH2 CH2 C ...
... 11 Ethers - Protecting Grps • the new C-C bond can be formed by alkylation of the acetylide anion • the OH group, however, is more acidic (pKa 16-18) than the terminal alkyne (pKa 25) • treating the compound with one mole of NaNH2 will give the alkoxide anion rather than the acetylide HC CCH2 CH2 C ...
Chapter 6 Addition Reactions to Alkenes
... University who won the Nobel Prize in 1977 for this work. The reaction proceeds by means of a one-step, concerted addition of the boron hydride to the alkene. Hydrogen is more electronegative than boron, so the electrophile in this reaction is boron. Note also that boron only has six electrons, so i ...
... University who won the Nobel Prize in 1977 for this work. The reaction proceeds by means of a one-step, concerted addition of the boron hydride to the alkene. Hydrogen is more electronegative than boron, so the electrophile in this reaction is boron. Note also that boron only has six electrons, so i ...
barker_rg
... Trimellitic-anhydride (the 1,2-anhydride of 1,2,4-tricarboxy benzene), hereafter referred to as TMA, contains both an aromatic acid group and a cyclic anhydride group. ...
... Trimellitic-anhydride (the 1,2-anhydride of 1,2,4-tricarboxy benzene), hereafter referred to as TMA, contains both an aromatic acid group and a cyclic anhydride group. ...
THE FRIEDEL-CRAFTS BENZYLATION OF ARENES AND THE
... opportunity to work in his research group. After the group arrived from UPenn, I was one of his first new PhD students at ETH and I feel really fortunate that I could join the group during this exciting time. Jeff, I want to thank you for all your support during the last 4 years, whether it was with ...
... opportunity to work in his research group. After the group arrived from UPenn, I was one of his first new PhD students at ETH and I feel really fortunate that I could join the group during this exciting time. Jeff, I want to thank you for all your support during the last 4 years, whether it was with ...
SUPPORTED LIGANDS FOR METAL CATALYZED REACTIONS Rocío Marcos Escartín ISBN:
... the most important processes in modern organic chemistry.[5] Classically, the Friedel-Crafts reaction,[6] the Diels-Alder reaction[7] and reactions related to the carbonyl group, such as addition of organometallic carbon nucleophiles[8] and aldol-type reactions (Mukaiyama aldol synthesis),[9] are ca ...
... the most important processes in modern organic chemistry.[5] Classically, the Friedel-Crafts reaction,[6] the Diels-Alder reaction[7] and reactions related to the carbonyl group, such as addition of organometallic carbon nucleophiles[8] and aldol-type reactions (Mukaiyama aldol synthesis),[9] are ca ...
Chapter 14 Aldehydes, Ketones, and Chiral Molecules
... Chapter 14 Aldehydes, Ketones and Chiral Molecules Oxidation and Reduction ...
... Chapter 14 Aldehydes, Ketones and Chiral Molecules Oxidation and Reduction ...
(omit), and Epoxides
... Name the OR group as an alkoxy substituent. • Common names: name the groups bonded to oxygen in alphabetical order followed by the word ether. ...
... Name the OR group as an alkoxy substituent. • Common names: name the groups bonded to oxygen in alphabetical order followed by the word ether. ...
Don`t forget to study the generic functional groups and the common
... 11. Write equations to show how the following transformation can be carried out. More than one step may be necessary. There are marks assigned for each intermediate product (not charged transition states) and there are marks for the reagents used; so list them all. No marks are assigned for mechanis ...
... 11. Write equations to show how the following transformation can be carried out. More than one step may be necessary. There are marks assigned for each intermediate product (not charged transition states) and there are marks for the reagents used; so list them all. No marks are assigned for mechanis ...
Alkenes and Alkynes I
... 15A. Syn Addition of Hydrogen: Synthesis of cis -Alkenes Semi-hydrogenation of alkynes to alkenes can be achieved using either the Ni2B (P-2) catalyst or the Lindlar’s ...
... 15A. Syn Addition of Hydrogen: Synthesis of cis -Alkenes Semi-hydrogenation of alkynes to alkenes can be achieved using either the Ni2B (P-2) catalyst or the Lindlar’s ...
Aldehydes, Ketones, & Chiral Molecules
... Has an IUPAC name in which the -e in the alkane name is changed to -al. Has a common name for the first four aldehydes that use the prefixes form (1C), acet (2C), propion (3C), and butyr (4C), followed by aldehyde. O O O ...
... Has an IUPAC name in which the -e in the alkane name is changed to -al. Has a common name for the first four aldehydes that use the prefixes form (1C), acet (2C), propion (3C), and butyr (4C), followed by aldehyde. O O O ...
Reactions of Alkenes
... Heats of Hydrogenation can be used to measure relative stability of isomeric alkenes correlation with structure is same as when heats of combustion are measured ...
... Heats of Hydrogenation can be used to measure relative stability of isomeric alkenes correlation with structure is same as when heats of combustion are measured ...
Project Overview
... Professor William Tam received his B.Sc. at the University of Hong Kong in 1990 and his Ph.D. at the University of Toronto (Canada) in 1995. He was an NSERC postdoctoral fellow at the Imperial College (UK) and at Harvard University (USA). He joined the Department of Chemistry at the University of Gu ...
... Professor William Tam received his B.Sc. at the University of Hong Kong in 1990 and his Ph.D. at the University of Toronto (Canada) in 1995. He was an NSERC postdoctoral fellow at the Imperial College (UK) and at Harvard University (USA). He joined the Department of Chemistry at the University of Gu ...
Chem. Soc. Rev., 2015, 44, 2202--2220 - RSC Publishing
... two potentially stereoselectivity-determining steps hidden in the catalytic cycle must be considered for asymmetric variants.23 The related hydrogenation is believed to pass through similar key intermediates as the hydrosilylation (cf. Scheme 1). Cooperative H–H activation by the Lewis acid catalyst ...
... two potentially stereoselectivity-determining steps hidden in the catalytic cycle must be considered for asymmetric variants.23 The related hydrogenation is believed to pass through similar key intermediates as the hydrosilylation (cf. Scheme 1). Cooperative H–H activation by the Lewis acid catalyst ...
Organic Chemistry
... in the electrophilic addition of HX to alkenes, so the product formed is the one with the halogen substituent upon the more highly substituted carbon. Also, rearrangement (hydride or methyl shift to form a more stable carbocation) might occur, typical of reactions that have a carbocation intermediat ...
... in the electrophilic addition of HX to alkenes, so the product formed is the one with the halogen substituent upon the more highly substituted carbon. Also, rearrangement (hydride or methyl shift to form a more stable carbocation) might occur, typical of reactions that have a carbocation intermediat ...
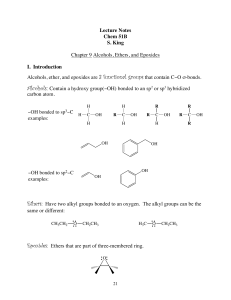
Lecture Notes Chem 51B S. King Chapter 9 Alcohols, Ethers, and
... An alcohol can undergo substitution if its OH group is converted into a group that is a better leaving group. Treatment of an alcohol with H-X converts the bad leaving group, −OH, into a great leaving group, −X. CH3OH + H ...
... An alcohol can undergo substitution if its OH group is converted into a group that is a better leaving group. Treatment of an alcohol with H-X converts the bad leaving group, −OH, into a great leaving group, −X. CH3OH + H ...
Acid-Catalyzed Hydration of Alkenes
... transition state for attack of water on bromonium ion has carbocation character; more stable transition state (left) has positive charge on more highly substituted carbon ...
... transition state for attack of water on bromonium ion has carbocation character; more stable transition state (left) has positive charge on more highly substituted carbon ...
Baylis–Hillman reaction

The Baylis–Hillman reaction is a carbon-carbon bond forming reaction between the α-position of an activated alkene and an aldehyde, or generally a carbon electrophile. Employing a nucleophilic catalyst, such as tertiary amine and phosphine, this reaction provides a densely functionalized product (e.g. functionalized allyl alcohol in the case of aldehyde as the electrophile). This reaction is also known as the Morita–Baylis–Hillman reaction or MBH reaction. It is named for the Japanese chemist Ken-ichi Morita, the British chemist Anthony B. Baylis and the German chemist Melville E. D. Hillman.DABCO is one of the most frequently used tertiary amine catalysts for this reaction. In addition, nucleophilic amines such as DMAP and DBU as well as phosphines have been found to successfully catalyze this reaction.MBH reaction has several advantages as a useful synthetic method: 1) It is an atom-economic coupling of easily prepared starting materials. 2) Reaction of a pro-chiral electrophile generates a chiral center, therefore an asymmetric synthesis is possible. 3) Reaction products usually contain multiple functionalities in a proximity so that a variety of further transformations are possible. 4) It can employ a nucleophilic organo-catalytic system without the use of heavy metal under mild conditions.Several reviews have been written.