Synthesis of a Family of Chiral Asymmetric Schiff - Blogs at H-SC
... resulted in oil, chromatography was performed on the first product. This yielded in sections of separations of solutions. Interestingly, the separations fluoresced under UV light, something not before catalogued in terms of chromatography. This led to the belief that clear separation had occurred, l ...
... resulted in oil, chromatography was performed on the first product. This yielded in sections of separations of solutions. Interestingly, the separations fluoresced under UV light, something not before catalogued in terms of chromatography. This led to the belief that clear separation had occurred, l ...
C1_5_products_from_oils_crossword
... 9. The reaction in which the enzymes in yeast turn glucose into ethanol and carbon dioxide. 10. An alkene with the formula C3H6. 11. Polymers that change in response to changes in their environment. 12. A hydrocarbon whose molecules contain at least one carbon-carbon double bond. Down 1. Something t ...
... 9. The reaction in which the enzymes in yeast turn glucose into ethanol and carbon dioxide. 10. An alkene with the formula C3H6. 11. Polymers that change in response to changes in their environment. 12. A hydrocarbon whose molecules contain at least one carbon-carbon double bond. Down 1. Something t ...
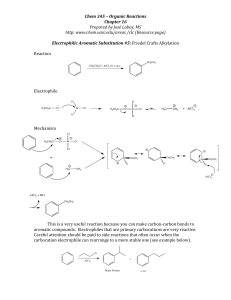
reactions of the carbonyl group in aldehydes and ketones
... A curly arrow is a symbol used in reaction mechanisms to show the movement of an electron pair in the braking or forming of a covalent bond ...
... A curly arrow is a symbol used in reaction mechanisms to show the movement of an electron pair in the braking or forming of a covalent bond ...
Slide 1
... • If the rate of direct addition is slowed down by steric hindrance, a Grignard reagent will form the conjugate addition product ...
... • If the rate of direct addition is slowed down by steric hindrance, a Grignard reagent will form the conjugate addition product ...
Slides for Chapter 1-4 - Department of Chemistry and Physics
... many alkyl halides(reaction as Lewis base) Nucleophiles that are Brønsted bases produce elimination ...
... many alkyl halides(reaction as Lewis base) Nucleophiles that are Brønsted bases produce elimination ...
Assignment 2 Group A and B
... 9) Which of the following alcohols can be prepared by the reaction of methyl formate with excess Grignard reagent? A) 1-pentanol B) 2-pentanol C) 3-pentanol D) 2-methyl-2-pentanol E) 3-methyl-3-pentanol 10) What reagent(s) would you use to accomplish the following conversion? ...
... 9) Which of the following alcohols can be prepared by the reaction of methyl formate with excess Grignard reagent? A) 1-pentanol B) 2-pentanol C) 3-pentanol D) 2-methyl-2-pentanol E) 3-methyl-3-pentanol 10) What reagent(s) would you use to accomplish the following conversion? ...
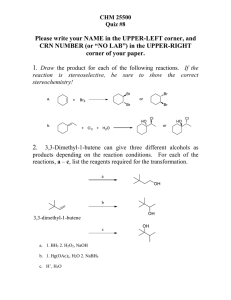
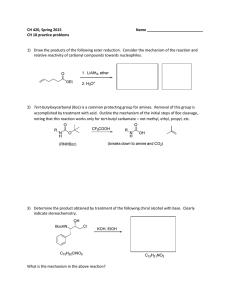
CH 420, Spring 2015 Name ___________________________ CH 18 practice problems
... 7) Rank the following compounds according to their relative acidity: cyclohexanol, phenol, pmethoxyphenol, p-nitrophenol. ...
... 7) Rank the following compounds according to their relative acidity: cyclohexanol, phenol, pmethoxyphenol, p-nitrophenol. ...
$doc.title
... Prepared by José Laboy, MS http://www.chem.wisc.edu/areas/clc (Resource page) Reactions of Alcohols #8: Reaction of a 1° Alcohol with Hydrogen Halides ...
... Prepared by José Laboy, MS http://www.chem.wisc.edu/areas/clc (Resource page) Reactions of Alcohols #8: Reaction of a 1° Alcohol with Hydrogen Halides ...
CHEM 201 Name Quiz 10 (Ch 17) ID Q1. Which of the following
... the ester shown below with LiAlH4? O O ...
... the ester shown below with LiAlH4? O O ...
Baylis–Hillman reaction

The Baylis–Hillman reaction is a carbon-carbon bond forming reaction between the α-position of an activated alkene and an aldehyde, or generally a carbon electrophile. Employing a nucleophilic catalyst, such as tertiary amine and phosphine, this reaction provides a densely functionalized product (e.g. functionalized allyl alcohol in the case of aldehyde as the electrophile). This reaction is also known as the Morita–Baylis–Hillman reaction or MBH reaction. It is named for the Japanese chemist Ken-ichi Morita, the British chemist Anthony B. Baylis and the German chemist Melville E. D. Hillman.DABCO is one of the most frequently used tertiary amine catalysts for this reaction. In addition, nucleophilic amines such as DMAP and DBU as well as phosphines have been found to successfully catalyze this reaction.MBH reaction has several advantages as a useful synthetic method: 1) It is an atom-economic coupling of easily prepared starting materials. 2) Reaction of a pro-chiral electrophile generates a chiral center, therefore an asymmetric synthesis is possible. 3) Reaction products usually contain multiple functionalities in a proximity so that a variety of further transformations are possible. 4) It can employ a nucleophilic organo-catalytic system without the use of heavy metal under mild conditions.Several reviews have been written.