oigchem
... Which type of systems act as E+ or Nu-? Same system act as E+ or Nu- depending on which system it reacts. Acidity and Basicity: General idea of order of acidity and basicity. Bronsted & Lewis theory. Effect of back-bonding , aromaticity, SIR,etc. ...
... Which type of systems act as E+ or Nu-? Same system act as E+ or Nu- depending on which system it reacts. Acidity and Basicity: General idea of order of acidity and basicity. Bronsted & Lewis theory. Effect of back-bonding , aromaticity, SIR,etc. ...
Chem 30BL_Lecture 2_.. - UCLA Chemistry and Biochemistry
... enthalpy (DH=23.9 kJ, ) nor the entropy (DS=84.91 J, ) changes much in the reaction and they also display opposing trends. Thus, the equilibrium constant is Keq=1.8 at 25 oC and Keq=8 at 80 oC, which are both low. ...
... enthalpy (DH=23.9 kJ, ) nor the entropy (DS=84.91 J, ) changes much in the reaction and they also display opposing trends. Thus, the equilibrium constant is Keq=1.8 at 25 oC and Keq=8 at 80 oC, which are both low. ...
Current Research Click Here
... Reagents for the oxidation of alcohols are often undesirable from an environmental point of view. Reagents such as PCC and the chemicals used in the Swern oxidation are not environmentally friendly. A recent report in the literature uses a Pd resin with the same functional group as the oxidation cat ...
... Reagents for the oxidation of alcohols are often undesirable from an environmental point of view. Reagents such as PCC and the chemicals used in the Swern oxidation are not environmentally friendly. A recent report in the literature uses a Pd resin with the same functional group as the oxidation cat ...
Chem 30BL * Lecture 2 - UCLA Chemistry and Biochemistry
... enthalpy (DH=23.9 kJ, ) nor the entropy (DS=84.91 J, ) changes much in the reaction and they also display opposing trends. Thus, the equilibrium constant is Keq=1.8 at 25 oC and Keq=8 at 80 oC, which are both low. ...
... enthalpy (DH=23.9 kJ, ) nor the entropy (DS=84.91 J, ) changes much in the reaction and they also display opposing trends. Thus, the equilibrium constant is Keq=1.8 at 25 oC and Keq=8 at 80 oC, which are both low. ...
Exam 2 SOLUTION
... The fluorines are more electronegative than the chlorines, and can better inductively withdraw electron density from the formed anion in the conjugate base. ...
... The fluorines are more electronegative than the chlorines, and can better inductively withdraw electron density from the formed anion in the conjugate base. ...
Honors Chemistry Organic Chemistry
... An unsaturated cis-fatty acid. An unsaturated trans-fatty acid. A saturated cis-fatty acid. A saturated trans-fatty acid. ...
... An unsaturated cis-fatty acid. An unsaturated trans-fatty acid. A saturated cis-fatty acid. A saturated trans-fatty acid. ...
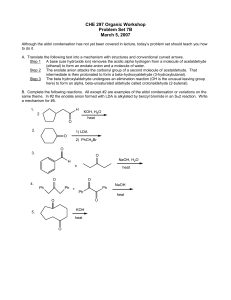
CHE 297 Organic Workshop
... (ethanal) to form an enolate anion and a molecule of water. Step 2 The enolate anion attacks the carbonyl group of a second molecule of acetaldehyde. That intermediate is then protonated to form a beta-hydroxyaldehyde (3-hydroxybutanal). Step 3 The beta hydroxylaldehyde undergoes an elimination reac ...
... (ethanal) to form an enolate anion and a molecule of water. Step 2 The enolate anion attacks the carbonyl group of a second molecule of acetaldehyde. That intermediate is then protonated to form a beta-hydroxyaldehyde (3-hydroxybutanal). Step 3 The beta hydroxylaldehyde undergoes an elimination reac ...
TYPES OF REACTIONS IN ORGANIC CHEMISTRY
... EVIDENCE FOR THE MECHANISM ~ the reaction will not occur in the dark at room temperature ~ the energy supplied is not enough to break the C - H bond ~ No molecular hydrogen produced - hence no hydrogen free radicals formed ~ Ethane is produced in small amounts, can only be explained by CH3 + CH3 ~ ...
... EVIDENCE FOR THE MECHANISM ~ the reaction will not occur in the dark at room temperature ~ the energy supplied is not enough to break the C - H bond ~ No molecular hydrogen produced - hence no hydrogen free radicals formed ~ Ethane is produced in small amounts, can only be explained by CH3 + CH3 ~ ...
Document
... of these reactions depend on whether the halogenoalkane is primary, secondary or tertiary. Explain the term nucleophilic substitution. ...
... of these reactions depend on whether the halogenoalkane is primary, secondary or tertiary. Explain the term nucleophilic substitution. ...
Unit 3 Goals - kimscience.com
... o explain how a catalyst speeds up a reaction in regards to activation energy. o draw the products of a dehydration synthesis reaction between an alcohol and a carboxylic acid, or between an amine and a carboxylic acid, and to explain how this type of reaction can be involved in creation of polymers ...
... o explain how a catalyst speeds up a reaction in regards to activation energy. o draw the products of a dehydration synthesis reaction between an alcohol and a carboxylic acid, or between an amine and a carboxylic acid, and to explain how this type of reaction can be involved in creation of polymers ...
Quiz 3 – Aldehydes and Ketones 1 Which of the following reactions
... 7 You have two C6H10O ketones, I and II. Both are optically active, but I is racemized by treatment with base and II is not. Wolff-Kishner reduction of both ketones gives the same achiral hydrocarbon, formula C6H12. What reasonable structures may be assigned to I and II? A) I is 3-methyl-4-penten-2- ...
... 7 You have two C6H10O ketones, I and II. Both are optically active, but I is racemized by treatment with base and II is not. Wolff-Kishner reduction of both ketones gives the same achiral hydrocarbon, formula C6H12. What reasonable structures may be assigned to I and II? A) I is 3-methyl-4-penten-2- ...
Nucleophilic Substitution Reaction
... In above mechanism the overall rate is limited to that of the slower second stage which depends only on the concentration of the conjugate base of the reactant. This mechanism is called as E1cB which means elimination, unimolecular, conjugate base. The distinction between E2 and E1cB mechanism can b ...
... In above mechanism the overall rate is limited to that of the slower second stage which depends only on the concentration of the conjugate base of the reactant. This mechanism is called as E1cB which means elimination, unimolecular, conjugate base. The distinction between E2 and E1cB mechanism can b ...
Elimination Reactions
... Draw a mechanism and energy diagram for elimination of an alcohol under acidic conditions Explain how additions of water to an alkene and elimination of an alcohol are opposite mechanisms Describe how to shift equilibrium in favor of elimination or addition Predict the major product accordin ...
... Draw a mechanism and energy diagram for elimination of an alcohol under acidic conditions Explain how additions of water to an alkene and elimination of an alcohol are opposite mechanisms Describe how to shift equilibrium in favor of elimination or addition Predict the major product accordin ...
... Cologne, Germany. His research interests include the synthesis of chiral ferrocene ligands, ligand atnchorage to inorganic supports, their application in stereoselective palladium- and rhodiumcatalysed reactions, and stereoselective synthesis on arenetricarbonylchromiumcomplexes. Stefan Toma is Prof ...
organic quiz 2
... the following will be the product(s)? a) 1-chlorohexane only b) 2-chlorohexane only c) 3-chlorohexane only d) both (b) and (c) 18) DNA is a natural polymer composed of a) glucose monomers b) nucleotide monomers c) amino acid monomers d) cellulose monomers 19) The process in which large organic molec ...
... the following will be the product(s)? a) 1-chlorohexane only b) 2-chlorohexane only c) 3-chlorohexane only d) both (b) and (c) 18) DNA is a natural polymer composed of a) glucose monomers b) nucleotide monomers c) amino acid monomers d) cellulose monomers 19) The process in which large organic molec ...
lec-2- 211(ES +Add)
... HX which can donate a proton, H2O should be able to add to alkenes in the same way as HBr, for example, resulting in the hydration of an alkene. However, for the addition of H2O to alkenes to occur acid catalysts are required. ...
... HX which can donate a proton, H2O should be able to add to alkenes in the same way as HBr, for example, resulting in the hydration of an alkene. However, for the addition of H2O to alkenes to occur acid catalysts are required. ...
Document
... • Imine formation is also a nucleophilic addition. • There is a different end result here, though as elimination of water occurs. • The initial reaction is attack of the amine on the carbonyl to give the alkoxide intermediate as normal. • Following protonation of the alkoxide and loss of the proton ...
... • Imine formation is also a nucleophilic addition. • There is a different end result here, though as elimination of water occurs. • The initial reaction is attack of the amine on the carbonyl to give the alkoxide intermediate as normal. • Following protonation of the alkoxide and loss of the proton ...
chemistry 2 - waiukucollegescience
... In order to distinguish between propan-1-ol and propene a student said it was necessary to use bromine water rather than acidified potassium permanganate. Discuss this statement. ...
... In order to distinguish between propan-1-ol and propene a student said it was necessary to use bromine water rather than acidified potassium permanganate. Discuss this statement. ...
Exam 2 Review A
... You should be familiar with the detailed mechanisms of the SN1 and SN2 reactions. Rate determining steps are important to consider, as are the transition states associated with these steps. Compare and contrast the SN1 and SN2 reactions with respect to kinetics, nature of the electrophile [structure ...
... You should be familiar with the detailed mechanisms of the SN1 and SN2 reactions. Rate determining steps are important to consider, as are the transition states associated with these steps. Compare and contrast the SN1 and SN2 reactions with respect to kinetics, nature of the electrophile [structure ...
Exam 2 Review A
... You should be familiar with the detailed mechanisms of the SN1 and SN2 reactions. Rate determining steps are important to consider, as are the transition states associated with these steps. Compare and contrast the SN1 and SN2 reactions with respect to kinetics, nature of the electrophile [structure ...
... You should be familiar with the detailed mechanisms of the SN1 and SN2 reactions. Rate determining steps are important to consider, as are the transition states associated with these steps. Compare and contrast the SN1 and SN2 reactions with respect to kinetics, nature of the electrophile [structure ...
What is an addition reaction
... In a condensation reaction, two organic molecules react together to produce one larger organic molecule and a molecule of water. For this type of reaction to occur, one of the molecules must have a hydroxyl group, and the other must have an active site with hydrogens, such as another hydroxyl group, ...
... In a condensation reaction, two organic molecules react together to produce one larger organic molecule and a molecule of water. For this type of reaction to occur, one of the molecules must have a hydroxyl group, and the other must have an active site with hydrogens, such as another hydroxyl group, ...
Exam 2 Review A
... You should be familiar with the detailed mechanisms of the SN1 and SN2 reactions. Rate determining steps are important to consider, as are the transition states associated with these steps. Compare and contrast the SN1 and SN2 reactions with respect to kinetics, nature of the electrophile [structure ...
... You should be familiar with the detailed mechanisms of the SN1 and SN2 reactions. Rate determining steps are important to consider, as are the transition states associated with these steps. Compare and contrast the SN1 and SN2 reactions with respect to kinetics, nature of the electrophile [structure ...
3.8 ADDITION OF WATER TO AN ALKENE H or enzyme + H-O
... Our example just shows one molecule of monomer as a reactant, although in fact there are large numbers of them that will react with each other to form the polymer: The one sided arrows indicate that one electron goes to each C atom. This is in contrast to the previous reaction pathways where both el ...
... Our example just shows one molecule of monomer as a reactant, although in fact there are large numbers of them that will react with each other to form the polymer: The one sided arrows indicate that one electron goes to each C atom. This is in contrast to the previous reaction pathways where both el ...
Baylis–Hillman reaction

The Baylis–Hillman reaction is a carbon-carbon bond forming reaction between the α-position of an activated alkene and an aldehyde, or generally a carbon electrophile. Employing a nucleophilic catalyst, such as tertiary amine and phosphine, this reaction provides a densely functionalized product (e.g. functionalized allyl alcohol in the case of aldehyde as the electrophile). This reaction is also known as the Morita–Baylis–Hillman reaction or MBH reaction. It is named for the Japanese chemist Ken-ichi Morita, the British chemist Anthony B. Baylis and the German chemist Melville E. D. Hillman.DABCO is one of the most frequently used tertiary amine catalysts for this reaction. In addition, nucleophilic amines such as DMAP and DBU as well as phosphines have been found to successfully catalyze this reaction.MBH reaction has several advantages as a useful synthetic method: 1) It is an atom-economic coupling of easily prepared starting materials. 2) Reaction of a pro-chiral electrophile generates a chiral center, therefore an asymmetric synthesis is possible. 3) Reaction products usually contain multiple functionalities in a proximity so that a variety of further transformations are possible. 4) It can employ a nucleophilic organo-catalytic system without the use of heavy metal under mild conditions.Several reviews have been written.