chemistry pretest - the Biology Scholars Program Wiki
... Below are 20 questions that represent some of the major concepts you have encountered in Chem105/205 (Chem120) and Chem211/212. Concepts surveyed in this pretest establish the foundations for the chemistry component of the biochemistry course. Since for most of you it has been a while since you have ...
... Below are 20 questions that represent some of the major concepts you have encountered in Chem105/205 (Chem120) and Chem211/212. Concepts surveyed in this pretest establish the foundations for the chemistry component of the biochemistry course. Since for most of you it has been a while since you have ...
Lecture6-Organometallic Chemistry
... • Principle of Microscopic Reversibility • If a certain series of steps constitutes the mechanism of a forward reaction, the mechanism of the reverse reaction is given by the same steps traversed backwards. (applies only to thermal reactions and not-photochemical reactions) • The sequence of transi ...
... • Principle of Microscopic Reversibility • If a certain series of steps constitutes the mechanism of a forward reaction, the mechanism of the reverse reaction is given by the same steps traversed backwards. (applies only to thermal reactions and not-photochemical reactions) • The sequence of transi ...
DEHYDRATION - ALKENE TEST EXERCISES
... DEHYDRATION - ALKENE TEST EXERCISES 1. Give a detailed mechanism for the acid-catalyzed dehydration of cyclohexanol to cyclohexene. ...
... DEHYDRATION - ALKENE TEST EXERCISES 1. Give a detailed mechanism for the acid-catalyzed dehydration of cyclohexanol to cyclohexene. ...
Organic Chemistry –Syllabus- one Semester Sackler faculty of
... double bond equivalent, alkyl group, Nomenclature (IUPAC rules), intermolecular forces( van der Waals force, Dipole–dipole interaction, Hydrogen bonds), Solubility, Conformations of alkanes(staggered-eclipsd) , Cycloalkanes, geometric isomers, The chair conformation of cyclohexane, Combustion of alk ...
... double bond equivalent, alkyl group, Nomenclature (IUPAC rules), intermolecular forces( van der Waals force, Dipole–dipole interaction, Hydrogen bonds), Solubility, Conformations of alkanes(staggered-eclipsd) , Cycloalkanes, geometric isomers, The chair conformation of cyclohexane, Combustion of alk ...
Organic Reactions 2.1- 2.3 - mccormack-sch4u-2013
... 4) OXIDATION & 5) REDUCTION REACTIONS • Change in the number of H or O atoms bonded to C • Always occur together • One reactant is oxidized while the other is reduced • For now, lets focus on reactant only… ...
... 4) OXIDATION & 5) REDUCTION REACTIONS • Change in the number of H or O atoms bonded to C • Always occur together • One reactant is oxidized while the other is reduced • For now, lets focus on reactant only… ...
Slide 1
... One needs to consider an alternative if there is another functional group present in the compound ...
... One needs to consider an alternative if there is another functional group present in the compound ...
Dehydration of 3,3-dimethyl-2-butanol to make alkenes March 1 & 3
... Do a GC analysis – Determine the % of your alkenes – Determine the % of your starting material (3,3-dimethyl-2butanol) ...
... Do a GC analysis – Determine the % of your alkenes – Determine the % of your starting material (3,3-dimethyl-2butanol) ...
chapter 2: reactions of organic compounds
... 4) OXIDATION & 5) REDUCTION REACTIONS • Change in the number of H or O atoms bonded to C • Always occur together • One reactant is oxidized while the other is reduced • For now, lets focus on reactant only… ...
... 4) OXIDATION & 5) REDUCTION REACTIONS • Change in the number of H or O atoms bonded to C • Always occur together • One reactant is oxidized while the other is reduced • For now, lets focus on reactant only… ...
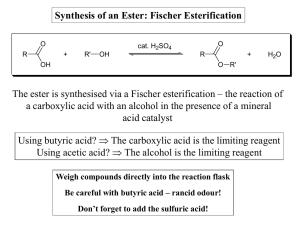
Synthesis of an Ester: Fischer Esterification The ester is synthesised
... The yield of the reaction is also dependent on the position of the equilibrium (i.e. it not lying completely in favour of products) A number of methods can be employed to drive the reaction completely to product, including: - Removing one of the products (ester or water) from the reaction, as it is ...
... The yield of the reaction is also dependent on the position of the equilibrium (i.e. it not lying completely in favour of products) A number of methods can be employed to drive the reaction completely to product, including: - Removing one of the products (ester or water) from the reaction, as it is ...
Calculating Percent Yield
... (Friedel-Crafts Alkylation) that incorporates the effect of substitution of the aromatic ring into the experiment. Students will prepare the product the first week of the experiment. During the second week, students will analyze the products by TLC analysis and melting point determination. In additi ...
... (Friedel-Crafts Alkylation) that incorporates the effect of substitution of the aromatic ring into the experiment. Students will prepare the product the first week of the experiment. During the second week, students will analyze the products by TLC analysis and melting point determination. In additi ...
organic chemistry ii
... Aldehydes and ketones which possess -hydrogens can undergo enolization. Most enols are unstable and reactive and instantly equilibrate to the “keto” form. Certain enols, such as -dicarbonyl compounds, among others, are exceptionally stable. Under basic conditions aldehydes and ketones form enolate ...
... Aldehydes and ketones which possess -hydrogens can undergo enolization. Most enols are unstable and reactive and instantly equilibrate to the “keto” form. Certain enols, such as -dicarbonyl compounds, among others, are exceptionally stable. Under basic conditions aldehydes and ketones form enolate ...
Chemistry 21 A - El Camino College
... 9. a) endothermic reaction is ___________________________________________________________________ b) exothermic reaction is ___________________________________________________________________ 10. The percentage yield is _____________________________________________________________________ __________ ...
... 9. a) endothermic reaction is ___________________________________________________________________ b) exothermic reaction is ___________________________________________________________________ 10. The percentage yield is _____________________________________________________________________ __________ ...
C h e m g u i d e ... ALCOHOLS: ESTERIFICATION
... 2. Draw the structures for the following esters, using a format like that in Q1 parts (a) and (b). a) methyl ethanoate b) propyl methanoate c) ethyl propanoate d) the ester (used as a banana flavour) formed by reacting 3-methylbutan-1-ol and ethanoic acid 3. a) You could make enough of the ester in ...
... 2. Draw the structures for the following esters, using a format like that in Q1 parts (a) and (b). a) methyl ethanoate b) propyl methanoate c) ethyl propanoate d) the ester (used as a banana flavour) formed by reacting 3-methylbutan-1-ol and ethanoic acid 3. a) You could make enough of the ester in ...
Chemistry 201 - Department of Chemistry | Oregon State University
... Which of the following statements is true? (A) a chiral molecule is not superimposable on its mirror image (B) glycine (Gly) is an amino acid which only has 1 chiral carbon (C) all amino acids are chiral (D) a chiral carbon has 3 identical groups bound to it (E) the following molecule is chiral: Cl ...
... Which of the following statements is true? (A) a chiral molecule is not superimposable on its mirror image (B) glycine (Gly) is an amino acid which only has 1 chiral carbon (C) all amino acids are chiral (D) a chiral carbon has 3 identical groups bound to it (E) the following molecule is chiral: Cl ...
Lecture 2 - UCLA Chemistry and Biochemistry
... This phenomenon -sometimes referred to as Evelyn effectcontradicts the fundamental principle in organic chemistry by which reactions always go by the lowest energy pathway. The 2-methylcyclohexanol provided is a mixture of cis and trans isomers (~47:53 by GC). For both isomers the conformer on the l ...
... This phenomenon -sometimes referred to as Evelyn effectcontradicts the fundamental principle in organic chemistry by which reactions always go by the lowest energy pathway. The 2-methylcyclohexanol provided is a mixture of cis and trans isomers (~47:53 by GC). For both isomers the conformer on the l ...
Aldehydes and ketones
... When some aldehyde is added to Tollens reagent and the mixture warmed, a redox reaction occurs. ...
... When some aldehyde is added to Tollens reagent and the mixture warmed, a redox reaction occurs. ...
1. Four of the structural isomers of C4H10O are alcohols. One of
... Calculate the maximum possible mass of butan-2-ol which could be obtained in the above experiment. ...
... Calculate the maximum possible mass of butan-2-ol which could be obtained in the above experiment. ...
Microsoft Word
... isolated yield. Benzoic anhydride was also used as the acylating species to yield the corresponding benzoates. Several primary, secondary, tertiary, and b-substituted alcohols were acylated by using 5-10 mol% of magnesium bromide as the catalyst (Scheme 8). Scheme 8. ...
... isolated yield. Benzoic anhydride was also used as the acylating species to yield the corresponding benzoates. Several primary, secondary, tertiary, and b-substituted alcohols were acylated by using 5-10 mol% of magnesium bromide as the catalyst (Scheme 8). Scheme 8. ...
Assignment 4 Task 1a
... have been assigned to a new case and are working as part of a team to solve the case. Working in the laboratory you will need to have a good understanding of the conventions adopted to ensure that all chemical compounds have unambiguous names. You also need to understand how a combination of element ...
... have been assigned to a new case and are working as part of a team to solve the case. Working in the laboratory you will need to have a good understanding of the conventions adopted to ensure that all chemical compounds have unambiguous names. You also need to understand how a combination of element ...
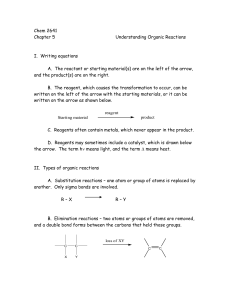
Chem 2641 Chapter 5 Understanding Organic Reactions I. Writing
... The initiation stage – Cl2 reacts with the uv light to form Cl. Propagation stage – The Cl. reacts with CH4 to form CH3. and HCl The CH3. reacts with Cl2 to form CH3Cl and Cl. Termination stage – any two radicals can combine to form a stable product. ...
... The initiation stage – Cl2 reacts with the uv light to form Cl. Propagation stage – The Cl. reacts with CH4 to form CH3. and HCl The CH3. reacts with Cl2 to form CH3Cl and Cl. Termination stage – any two radicals can combine to form a stable product. ...
benzylic alcohols
... Electron-withdrawing substituents decrease the yields and orthosubstituensts hinder the reaction e.g. neither 2, 4 dinitro-nor 2, 6 dimethyl benzaldehyde can be prepared in this way. The reaction involves hydride –ion transfer. At the acidity employed, the quaternary benzyl salt is hydrolyzed to the ...
... Electron-withdrawing substituents decrease the yields and orthosubstituensts hinder the reaction e.g. neither 2, 4 dinitro-nor 2, 6 dimethyl benzaldehyde can be prepared in this way. The reaction involves hydride –ion transfer. At the acidity employed, the quaternary benzyl salt is hydrolyzed to the ...
- EdShare - University of Southampton
... Alkenes are unsaturated compounds that can be used in organic synthesis. They can be formed in elimination reactions of halogenoalkanes. An example of this is the reaction between 2-bromopentane and hot ethanolic KOH. Using your knowledge of reaction mechanisms, draw appropriate curly arrows to comp ...
... Alkenes are unsaturated compounds that can be used in organic synthesis. They can be formed in elimination reactions of halogenoalkanes. An example of this is the reaction between 2-bromopentane and hot ethanolic KOH. Using your knowledge of reaction mechanisms, draw appropriate curly arrows to comp ...
Baylis–Hillman reaction

The Baylis–Hillman reaction is a carbon-carbon bond forming reaction between the α-position of an activated alkene and an aldehyde, or generally a carbon electrophile. Employing a nucleophilic catalyst, such as tertiary amine and phosphine, this reaction provides a densely functionalized product (e.g. functionalized allyl alcohol in the case of aldehyde as the electrophile). This reaction is also known as the Morita–Baylis–Hillman reaction or MBH reaction. It is named for the Japanese chemist Ken-ichi Morita, the British chemist Anthony B. Baylis and the German chemist Melville E. D. Hillman.DABCO is one of the most frequently used tertiary amine catalysts for this reaction. In addition, nucleophilic amines such as DMAP and DBU as well as phosphines have been found to successfully catalyze this reaction.MBH reaction has several advantages as a useful synthetic method: 1) It is an atom-economic coupling of easily prepared starting materials. 2) Reaction of a pro-chiral electrophile generates a chiral center, therefore an asymmetric synthesis is possible. 3) Reaction products usually contain multiple functionalities in a proximity so that a variety of further transformations are possible. 4) It can employ a nucleophilic organo-catalytic system without the use of heavy metal under mild conditions.Several reviews have been written.