* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Steric protection of alkylidene is not needed:
Kinetic resolution wikipedia , lookup
Elias James Corey wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Aromaticity wikipedia , lookup
Asymmetric hydrogenation wikipedia , lookup
Asymmetric induction wikipedia , lookup
Discodermolide wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Hydrogenation wikipedia , lookup
Persistent carbene wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Stille reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Ene reaction wikipedia , lookup
Fischer–Tropsch process wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
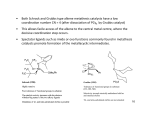
M=CH2 carbenes: (also M=CH2 species can be isolated) The bimolecular reaction of M=CH2 to ethylene species is one of the major pathways for decomposition of metathesis catalysts! First Metal-Carbenes where not active in metathesis: Coincidental discovery of active W-Carbene: Active in Metathesis (only with minor amounts of AlCl3) Design of active W-Carbene catalyst: - carbene should be neopentylidene (quite stable) - complex should be well-accessible for alkenes (low coordination of 4 => free coordination site) - X should NOT be O (dimer formation) but NR with large R group - other substituents should be electron-withdrawing (important for alkene coordination) Synthesis: Problems with W-Carbene catalyst: - Reactions sometimes slow - Intermediate can be isolated Mo-Carbene catalysts (Remember: bonds to first-row transition metals are weaker = more dynamic) Selective Cross Metathesis: Aim = selectively obtain product R1CHCHR2 Alkyne metathesis: Nitrile-Alkyne cross metathesis: Ring opening metathesis: (ROM) Ring closure metathesis: (RCM) (Grubbs, JACS 1992) Mechanism ring closure metathesis: - Reaction is driven by entropy: 1 molecule => 2 molecules Even 7-membered rings are possible By-product is easily removable gas No polymer by-products: intramolecular reactions are faster than intermolecular reactions - Also heavily substituted olefins can be converted Nowadays metathesis catalysis is extremely important in organic synthesis, especially for making ring compounds! ADMET: (acyclic diene metathesis) polymers Ring opening metathesis polymerization: (ROMP) Mechanism: ROMP with chiral enantioselective catalysts: isotactic Chiral enantioselective catalysts: Grubbs catalysts: - coincidence; it was planned to polymerize the cyclopropene - Cl2(PPh3)2Ru=CH-CH=CPh2 is not very active in metathesis but Cl2(PCy3)2Ru=CH-CH=CPh2 is! - BIG ADVANTAGE: not air-sensitive (reactions can be run in air) - Disadvantage: large scale synthesis not possible... 1st generation Grubbs catalyst: - One-pot synthesis From Cl2Ru(PCy3)2 + PhCHN2 + P(Cy)3 Multi-kg scale! Fundamental difference with Schrock carbene: electron-rich metal that likes soft ligands like ethylene but not Lewis-bases like H2O - => water and functional group tolerant 2nd generation Grubbs catalyst: - substitution of PCy3 for Arduengo Carbene (highly electrondonating) Ru even more electron rich => highly active catalyst (very functional group tolerant!) Self-healing polymers Grubbs, Nature 2001, 409, 794. Cross-linked dicyclopentadiene polymer: First proposed mechanism for metathesis: (Calderon) Bis-olefine mechanism (even number of carbon atoms): Chauvin mechanism for metathesis: M-Carbene mechanism (odd number of carbon atoms): .Exchanging dance partners Grubbs experiment to prove mechanism: FOUND: complete scrambling of deuterium