* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download - University at Albany
George S. Hammond wikipedia , lookup
Discodermolide wikipedia , lookup
Asymmetric induction wikipedia , lookup
Hydrogenation wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Hydroformylation wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
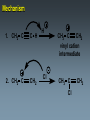
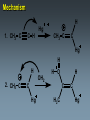
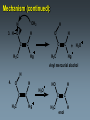
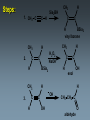
Alkynes State University of New York at Albany Alkynes: Are hydrocarbons that contain carbon-carbon triple bonds. Are also known as acetylenes. Have the general structural formula CnH2n-2. Are similar in physical properties to alkanes and alkenes. They are nonpolar and water insoluble. The triple bond is shorter than C=C and C-C bonds with a length of 1.20 Å. The most important commercial alkyne is acetylene, which is used as a fuel. Nomenclature of Alkynes Similar to that of alkenes, except the ending of the root name corresponding to the longest continuous chain containing the triple bond is changed from “ene” to “yne”. E.g. ethene becomes ethyne, propene becomes propyne. The chain is numbered from the end closest to the triple bond. When a double and a triple bond is present, the compound is named as an “alkenyne”. When a triple bond and an OH are present, the compound is named as an alkynol. Examples: H C C H H C ethyne (acetylene) H2C C C C CH3 H3C propyne C C CH3 H3C CH3 2-butyne CH3 CH3 C CH CH3 C C CH2 CH CH3 6-bromo-2-ethyl-3-pentyne 4-methyl-1-penten-2-yne Common names: H C C acetylene H Ph C C Ph diphenylacetylene Ph C C Ph ethylmethylacetylene Alkynes can be described as internal or terminal. If the triple bond is between two carbons, the alkyne is internal. If it is flanked by a hydrogen and a carbon, it is a terminal alkyne. The terminal hydrogen of a terminal alkyne is called the acetylenic hydrogen. In general, internal alkynes are more stable than terminal alkynes. H3C C C CH3 2-butyne an internal alkyne CH3 CH2 C C 1-butyne a terminal alkyne H acetylenic hydrogen Acidity of Alkynes Recall that the amount of s character of sp, sp2, and sp3 hybrid orbitals is , and respectively. As the s character increases, so does the acidity. Therefore, acetylenic protons are relatively acidic, with a pKa of ~ 25. Acetylenic protons are more acidic than vinyl (pKa = 44) or alkane (pka = 50) protons. Strong bases such as -NH (but not alkoxide and hydroxide ions) can 2 deprotonate alkynes. Internal alkynes do not have acetylenic protons, so they cannot undergo this type of deprotonation. Acidity of Alkynes—Deprotonation. When terminal alkynes are deprotonated by very strong base, the resulting anion is called an acetylide ion. Deprotonation of terminal alkynes is commonly done with NH2, in the form of NaNH2 (sodium amide or “sodamide”). Acetylide ions are strong nucleophiles. from NaNH2 CH3 CH2 C C H NH2 CH3 CH2 C C Na sodium acetylide (sodium butynide) Acidity of Alkynes—Heavy Metal Acetylides. Silver (I) and copper (I) salts react with terminal alkynes to form silver and copper acetylides. Silver and copper acetylides are much less basic and less nucleophilic than their sodium counterpart. Thus, they don’t have much synthetic utility. Silver and copper acetylides are not very soluble, and so they form characteristic precipitates. This forms the basis for a simple chemical test for terminal alkynes. Internal alkynes do not react and form a precipitate because they don’t have an acidic proton. Examples: CH3 CH2 C C H Ag CH3 CH2 C C Ag a light colored precipitate CH3 CH2 C C H Cu CH3 CH2 C C Ag a brick red precipitate H3C C C CH3 Ag or Cu No reaction Treatment of the silver or copper acetylide with acid (e.g. HCl) yields the original alkyne. Synthesis of Alkynes 1. Alkylation of Acetylide Ions: Acetylide ions react with primary or methyl halides via an SN2 mechanism to produce elongated alkynes. If 2o or 3o alkyl halides are used, the acetylide ion may act as a base, yielding an alkene by an E2 mechanism. H 1. NaNH2 CH2CH3 2. CH3CH2Br Br CH3 CH2 C C + E2 CH3 CH2 C C H + Synthesis of Alkynes 2. Addition of Acetylide Ions to carbonyl groups: A carbonyl is a C=O group. Because the C=O is polarized, there is a + charge on carbon, and a - charge on oxygen. Therefore, the carbonyl carbon iselectrophilic. Acetylide ions can attack carbonyl carbonsto give alkoxide ions which when protonated, yield alcohols. CH3 CH3 CH3 C C C O CH3 C C C H H CH3 CH3 C C C H CH3 O H CH3 C C C OH H acetylenic alcohol O Synthesis of Alkynes 3. Synthesis of Alkynes by Elimination Reactions: Elimination of two molecules of HX from a geminal or vicinal dihalide yields an alkyne. When the base used is KOH, the product formed is the internal alkyne. When the base used is NaNH2, the terminal alkyne is formed. Cl KOH, 200 oC CH3CH2 C C CH3 Cl Cl NaNH2, 150 oC Cl CH3CH2CH2 C C H Addition Reactions of Alkynes Reagents add to the triple bond of alkynes just as they add across the double bond of alkenes. Since alkynes have two triple bonds, up to two molecules can add across the triple bond, depending upon the reagents and conditions. 1a. Reduction to Alkanes: Hydrogen adds to alkynes in the presence of a catalyst to form alkanes. H CH3CH2 C C CH3 Pt, Pd, or Ni CH3CH2 C H H C CH3 H Addition Reactions of Alkynes 1b. Hydrogenation to cis Alkenes: Cis alkenes can be formed from alkynes by using Lindlars catalyst; internal alkynes yield the cis-product. Lindlar's catalyst CH3CH2 C C CH3 H2, Pd/BaSO4 quinoline, CH3OH Addition Reactions of Alkynes 1c. Metal-Ammonia Reduction to trans Alkenes: Treatment of alkynes with sodium in liquid ammonia generates alkynes with a trans orientation. CH3CH2 C C CH3 Na/NH3 2. Addition of Halogens: Br2 and Cl2 add to alkynes just as they add to alkenes. If the alkene forms, there is often a mixture of cis and trans isomers. Often the reaction proceeds all the way to the tetrahalide. Br CH3CH2 C C CH3 Br2 + Br 72% Br Br 28% Addition of HX to Alkynes HX adds to alkynes just as it does to alkenes. When HX is added to terminal alkynes, Markovnikov orientation is observed. When two moles of HX are added, a geminal dihalide is formed. In the addition of HBr in the presence of peroxides, anti-Markovnikov orientation is observed. Examples: CH3 C C H HCl CH3 C CH2 Cl CH3 C C H 2 HCl Cl CH3 C Cl CH3 Mechanism 1. CH3 C C H H CH3 C CH2 vinyl cation intermediate 2. CH3 C CH2 Cl CH3 C Cl CH2 Hydration of Alkynes to Aldehydes and Ketones 1. Mercuric Ion Catalyzed Hydration: In the presence of H3O+ and mercuric ion catalyst, water adds across the triple bond with Markovnikov orientation. Aldehydes and ketones are produced in the process. H CH3 C C H HgSO4 H H2SO4 H OH a vinyl alcohol (enol) O Mechanism H Hg 1. CH3 C C H CH3 C C Hg H H 2. CH3 C H O H OH2 C C Hg H3C C Hg Mechanism (continued): OH2 H 3. H O H H C O C H3C H C Hg C H3C + H3O Hg vinyl mercurial alcohol H 4. O H C H3C HO H3O C Hg H C H3C C H enol Mechanism (continued): Vinyl alcohols (enols) are very unstable, and isomerize to the ketone form. This type of rapid equilibrium is called tautomerism. Tautomerism is NOT resonance! HO H C H3C HO H C H enol H C C H3C H H keto-enol tautomerism H O H C H3C C O H H OH2 C H3C CH3 Hydroboration-Oxidation of Alkynes Hydroboration of alkynes with diisoamyl borane results in the formation of a vinyl alcohol with anti-Markovnikov orientation. This vinyl alcohol then tautomerizes to an aldehyde. H CH3 C C H 1. Sia2BH 2. H2O2/OH CH3 CH2 C O Steps: Sia2BH 1. CH3 C CH3 C H H C C H BSia2 vinyl borane CH3 C 2. C H C NaOH C H BSia2 CH3 H H2O2 C H 3. CH3 H OH enol H H OH C CH3 CH2 C OH O aldehyde Permanganate Oxidation of Alkynes Treatment of alkynes with permanganate under neutral conditions results in the formation of -diketones. CH3CH2 C C CH3 KMnO4 H2O, neutral CH3CH2 C C CH3 O O 2,3-pentanedione Permanganate Oxidation of Alkynes If the reaction mixture becomes too warm or too basic, the diketone undergoes oxidative cleavage to form salts of carboxylic acids CH3CH2 C C CH3 KMnO4/KOH H2O, heat CH3CH2 C O + O C CH3 O O HCl/H2O CH3CH2 C OH + HO O C CH3 O Permanganate Oxidation of Alkynes Terminal alkynes are cleaved similarly to give a carboxylic acid and CO2. CH3CH2 C C H 1. KMnO4/KOH H2O, heat CH3CH2 C OH + CO2 2. H O Ozonolysis of Alkynes Ozonolysis of an alkyne gives products similar to those obtained from oxidative cleavage by permanganate. CH3CH2 C C CH3 1. O 3 2. H2O CH3CH2 C OH + HO C O O CH3