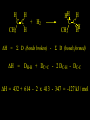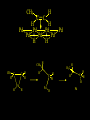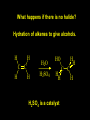* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Reactions
Kinetic resolution wikipedia , lookup
Discodermolide wikipedia , lookup
Elias James Corey wikipedia , lookup
Marcus theory wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Asymmetric induction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Stille reaction wikipedia , lookup
Ene reaction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
George S. Hammond wikipedia , lookup
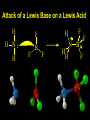
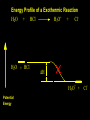
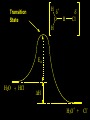
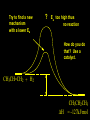
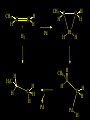
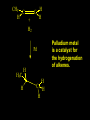
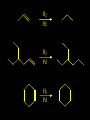
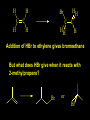
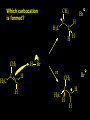
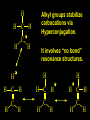
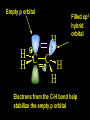
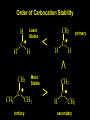
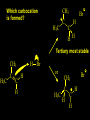
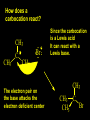
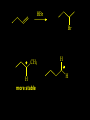
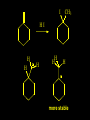
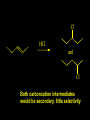
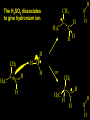
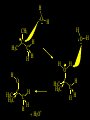
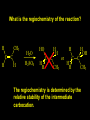
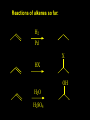
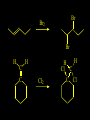
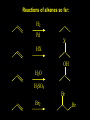
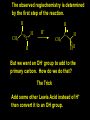
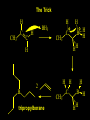
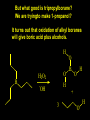
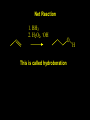
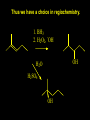
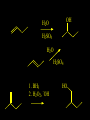
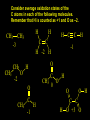
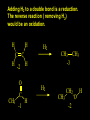
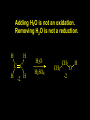
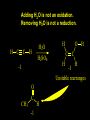
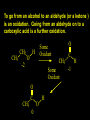
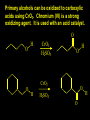
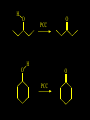
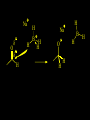
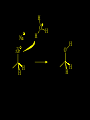
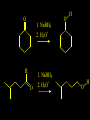
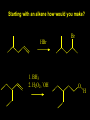
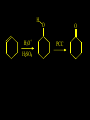
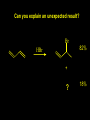
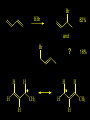
Reactions and Reaction Mechanisms A reaction mechanism shows the actual flow of electrons and movement of the atoms during the reaction. If one can understand a reaction mechanism one can predict the course of other reactions. Attack of a Lewis Base on a Lewis Acid H F H N B H F H F H H F N B F F It is also useful to correlate changes in energy with the movement of atoms. To do this one creates an energy profile of the reaction showing how the energy changes as the atoms move. Energy Profile of a Exothermic Reaction H2O + H2O + HCl H3O+ HCl + Cl- H + H3O + Potential Energy Cl - H + O H Transition State H Cl Ea H2O + HCl H H3O+ + Cl- H H C C + CH3 H H = H H2 D (bonds broken) - = HH H C C H CH3 H D (bonds formed) DH-H + DC=C - 2 DC-H - DC-C H = 432 + 614 - 2 x 413 - 347 = -127 kJ / mol H H C C + CH3 H H2 HH H C C H CH3 H But if you mix propene and H2 nothing happens! H H CH3 H C C H H Molecules repel each other. No good mechanism for the reaction. Try to find a new mechanism with a lower Ea ? Ea too high thus no reaction How do you do that? Use a catalyst. CH3CH=CH2 + H2 CH3CH2CH3 H = -127kJ/mol Palladium metal can be used as a catalyst for the hydrogenation of alkenes. H2 will actually dissolve in Pd metal. The H atoms dissociate and go into the octahedral holes. The p bond of an alkene will bond to the surface atom of the Pd metal. This is all we need! CH3 C C H H Pd H Pd Pd Pd Pd Pd H H CH3 H CH3 C H H C H H Pd H Pd H3C H C H H Pd H H H C H H H Pd CH3 C H H3C H + H2 CH3 C H C H H Pd H C H H Pd H H CH3 H H H C H H Pd C H H Pd H CH3 C H + H2 C H H Pd H3C H H H C H H Palladium metal is a catalyst for the hydrogenation of alkenes. H2 Pd H2 Pd H2 Pd H H C C H HH Br C C HH H H Addition of HBr to ethylene gives bromoethane But what does HBr give when it reacts with 2-methylpropene? Br or Br But what does HBr give when it reacts with 2-methylpropene? Br or Br For this we need to know the mechansism. The reaction involves a carbocation intermediate. Which carbocation is formed? CH3 H3C C Br C H H H H Br CH3 H3C C C H H or H3C Br CH3 C H C H H H Alkyl groups stabilize carbocations via Hyperconjugation. H C H C H H It involves “no bond” resonance structures. H H H H C H H C H H C H C C C H H H H H H Empty p orbital H Filled sp3 hybrid orbital H C C H H H Electrons from the C-H bond help stabilize the empty p orbital Order of Carbocation Stability Least Stable H H C H CH3 < H C primary H < CH3 C CH3 tertiary < CH3 Most Stable CH3 H C CH3 secondary Which carbocation is formed? CH3 H3C C Br C H H H Tertiary most stable H Br CH3 H3C C C H H or H3C Br CH3 C H C H H How does a carbocation react? CH3 CH3 C Br Since the carbocation is a Lewis acid It can react with a Lewis base. CH3 The electron pair on the base attacks the electron deficient center CH3 CH3 C Br CH3 General Reaction: The addition of a hydrogen halide, HCl, HBr or HI to an alkene gives an alkyl halide. The regiochemistry is determined by which carbocation is the most stable. HBr Br CH3 H more stable H H I CH3 HI H H H H H H more stable Cl HCl and Cl Both carboncation intermediates would be secondary, little selectivity What happens if there is no halide? Hydration of alkenes to give alcohols. H H C C H H H2O H2SO4 HO H H H2SO4 is a catalyst C C H H H What is the regiochemistry of the reaction? H CH3 C H C H H2O H2SO4 HO H H C C H H H or CH3 H H C C What is the mechanism of the reaction? H OH CH3 H CH3 The H2SO4 dissociates to give hydronium ion. H3C C O C H H H H H H O CH3 H3C C C H H H or H3C CH3 C H C H H H O H H O H H CH3 H3C C C O H H H H H H O H3C C C H H3C H H O H H3C C C H H3C H H + + H3O What is the regiochemistry of the reaction? H CH3 C H C H H2O H2SO4 HO H H C C H H H or CH3 H H C C H OH CH3 The regiochemistry is determined by the relative stability of the intermediate carbocation. Reactions of alkenes so far: H2 Pd X HX OH H2O H2SO4 One more reaction for the sake of completeness Br Br2 Br Bromine or chlorine easily add to a double bond to give dibromo or dichloro compounds. Br Br2 Br H C C H Cl2 H H Cl C C Cl Reactions of alkenes so far: H2 Pd X HX OH H2O H2SO4 Br Br2 Br Enough of this simple stuff. The acid catalyzed addition of water to an alkene gives an alcohol. OH H2O H2SO4 The regiochemistry is determined by the relative stability of the intermediate carbocation. But what if you want 1-propyl alcohol? What could you do to “trick” the regiochemistry? The observed regiochemistry is determined by the first step of the reaction. H CH3 C H C H H+ CH3 C H HH H But we want an OH- group to add to the primary carbon. How do we do that? The Trick Add some other Lewis Acid instead of H+ then convert it to an OH group. The Trick H CH3 C H C BH3 H CH3 C B H H H H H B H 2 tripropylborane CH3 H H C H B H H H But what good is tripropylborane? We are tryingto make 1-propanol? It turns out that oxidation of alkyl boranes will give boric acid plus alcohols. H B H2O2 O - H OH O B O H + 3 O H Net Reaction 1. BH3 2. H2O2, -OH This is called hydroboration O H Thus we have a choice in regiochemistry. 1. BH3 2. H2O2, -OH OH H2O H2SO4 OH OH H2O H2SO4 H2O H2SO4 1. BH3 2. H2O2, -OH HO Oxidation states in carbon chemistry. Oxidation and reduction are important chemical processes. In organic chemistry it is not always obvious that you are carrying out an oxidation or a reduction. Consider average oxidation states of the C atoms in each of the following molecules. Remember that H is counted as +1 and O as –2. H CH3 CH3 -3 CH3 CH2 -2 O H C C C H -2 H C -1 C H -1 O H C H CH3 O 0 O CH3 H H O O H C C H O +3 O Adding H2 to a double bond is a reduction. The reverse reaction ( removing H2) would be an oxidation. H H H2 CH3 CH3 -3 C C H -2 H O CH3 C -1 H2 H CH3 CH2 -2 O H Adding H2O is not an oxidation. Removing H2O is not a reduction. H H C C H -2 H H2O H2SO4 CH3 CH2 -2 O H Adding H2O is not an oxidation. Removing H2O is not a reduction. H C C H H2O H2SO4 -1 H O H C H C -1 H Unstable rearranges O CH3 C -1 H To go from an alcohol to an aldehyde (or a ketone ) is an oxidation. Going from an aldehyde on to a carboxylic acid is a further oxidation. CH3 CH2 O H CH3 -2 Some Oxidant O CH3 O Some Oxidant C 0 O H C -1 H Primary alcohols can be oxidized to carboxylic acids using CrO3. Chromium (VI) is a strong oxidizing agent. It is used with an acid catalyst. O O H CrO3 H2SO4 O CrO3 O H H O H2SO4 O H Alcohols can be oxidized to aldehydes or ketones using a modified form of CrO3 called PCC for pyridinium chlorochromate, (C5H6NCrO3Cl). It is a milder reagent and if you use it carefully you can stop the reaction at the intermediate aldehyde step. O PCC O H H H O O PCC O H O PCC Going Backwards: Reduction of aldehydes or ketones to an alcohol. To reduce a carbonyl group, C=O, to an alcohol one could use H2 gas, but a simpler way is to use a hydride as a source of H- and a acid as a source of H+. O 1. NaBH4 2. H3O+ The reagent of choice is NaBH4, followed by some acid. O H Na H B H H H O H H Na H O H H B H H Na O O H H O H H H H H O 1. NaBH4 + 2. H3O H O 1. NaBH4 + 2. H3O O H O H Starting with an alkene how would you make? HBr 1. BH3 2. H2O2, OH Br O H H H3O+ H2SO4 O O PCC Can you explain an unexpected result? Br 82% HBr + ? 18% Br HBr 82% and Br H ? H H H CH3 H 18% H H CH3 H