* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Document 8195609
Survey
Document related concepts
Homoaromaticity wikipedia , lookup
Discodermolide wikipedia , lookup
Bottromycin wikipedia , lookup
Elias James Corey wikipedia , lookup
Kinetic resolution wikipedia , lookup
Asymmetric induction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Petasis reaction wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Hydroformylation wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Transcript
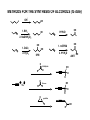
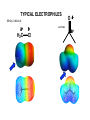
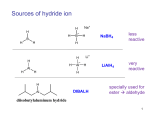
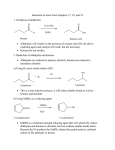
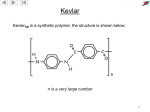
METHODS FOR THE SYNTHESIS OF ALCOHOLS (to date) OH I - OH 1. BH3 OH H+/H2O OH 2. NaOH/H2O2 1. OsO4 2. H2O2 OH OH 1. mCPBA OH 2. H+/H2O SYN O Aldehydes OH H R ANTI H R O Na OH Ketones R R R R O epoxides OH R R OH ELECTROPHILIC AND NUCLEOPHIC CARBON Methylmagnesium bromide d- H3 C d+ Mg Br Methyl lithium Methyl chloride d- d+ d+ H3 C Li H3C d- Cl TYPICAL ELECTROPHILES Methyl chloride d+ H3C d- Cl acetone O dd+ Alcohols, carbonyl compounds and carboxylic acids: REDUCTION Reduction: Addition of H2 (or H-), loss of O or O2 ; loss of X2 H H R C R H C OH H Primary alcohol H O NaBH4 R C or H Raney Ni/ H2 Aldehyde O LiAlH4 R C OH Carboxylic acid must convert OH to leaving group H H R C H R C OH R' Secondary alcohol R' must convert OH to leaving group R C R' H R or Raney Ni/ H2 R C R' Ketone H Li H Al H R" R" O NaBH4 C OH R' Tertiary alcohol H Lithium aluminium hydride Na H H B H H Sodium borohydride Comparison of Reducing Agents • LiAlH4 is stronger. • LiAlH4 reduces more stable compounds which are resistant to reduction. => Alcohols, carbonyl compounds and carboxylic acids: OXIDATION Oxidation: loss of H2 , addition of O or O2, addition of X2 (halogens) H H R C H R H H C R OH H R C OH R H2 SO4 C OH Carboxylic acid O R C H2 SO4 R' Secondary alcohol R' C Aldehyde Na2Cr2O7 / O Na2Cr2O7 / H H Primary alcohol H R C O PCC X R' Ketone PCC R" R" R C R' H R C OH R' Tertiary alcohol X NH CrO3Cl-