* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Oxidation of Alcohols
Survey
Document related concepts
Transcript
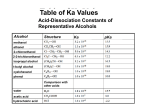
Oxidation of Alcohols By Iona and Catherine Oxidising Agents • Primary and secondary alcohols can be oxidised using an oxidising agent, notated by [o]. • A suitable oxidising agent is a solution containing acidified dichromate ions (H+ and Cr O 2-). • These ions come from a mixture of K Cr O and sulphuric acid. • During the reaction there will be a colour change of orange to green. 2 7 2 2 7 Primary Alcohol • With gentle heating, a primary alcohol can be oxidised to produce an aldehyde. • With strong heating and excess [o] a carboxylic acid is formed. • Reflux apparatus is generally used to produce carboxylic acids. • Aldehydes must be distilled as they are formed to prevent further oxidation which may form carboxylic acids. • • Aldehydes have a C=O bond at the end of a carbon chain Carboxylic acids have a C=O and C-OH group. Secondary Alcohols • Secondary alcohols are oxidised to produce ketones, they cannot be oxidised further! • Secondary alcohols are oxidised with gentle heat and the same oxidising agent. • Ketones have a C=O bond in the middle of a carbon chain. Tertiary Alcohols • These are resistant to oxidation, there will be no colour change. • This is because the carbon which the alcohol group is bonded to is not bonded to any other hydrogen atoms and so no double bonds can be created.