* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Neural correlates of incidental and directed facial emotion
Neuropsychology wikipedia , lookup
Neuroplasticity wikipedia , lookup
Biology of depression wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Time perception wikipedia , lookup
Optogenetics wikipedia , lookup
Executive functions wikipedia , lookup
Decision-making wikipedia , lookup
Child Lying wikipedia , lookup
Emotion and memory wikipedia , lookup
Neurolinguistics wikipedia , lookup
Metastability in the brain wikipedia , lookup
Neuromarketing wikipedia , lookup
Functional magnetic resonance imaging wikipedia , lookup
Embodied cognitive science wikipedia , lookup
Sex differences in cognition wikipedia , lookup
History of neuroimaging wikipedia , lookup
Neuroeconomics wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Limbic system wikipedia , lookup
Embodied language processing wikipedia , lookup
Impact of health on intelligence wikipedia , lookup
Face perception wikipedia , lookup
Neurophilosophy wikipedia , lookup
Aging brain wikipedia , lookup
Neuroesthetics wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Anterior cingulate cortex wikipedia , lookup
Affective computing wikipedia , lookup
Emotion perception wikipedia , lookup
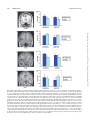
doi:10.1093/scan/nsp029 SCAN (2009) 4, 387–398 Neural correlates of incidental and directed facial emotion processing in adolescents and adults Alessandra M. Passarotti,1,2 John A. Sweeney,2 and Mani N. Pavuluri1,2 1 Institute for Juvenile Research, and 2Center for Cognitive Medicine, Department of Psychiatry, University of Illinois at Chicago, Chicago, IL, USA Keywords: functional magnetic resonance imaging (fMRI); face emotion; emotion; incidental; development; adolescent INTRODUCTION A developmental milestone for social communication and interactions is the ability to regulate one’s own emotional reactions in order to produce socially appropriate behaviors (Adolphs, 2002; Bell and Deater-Deckard, 2007). There is some consensus on the view that better emotional selfregulation in late adolescence and adulthood may result from improved executive functions and cognitive control over affect processes (Posner and Rothbart, 2000; Monk et al., 2003; Nelson et al., 2005; Blakemore 2008). In fact, recent studies have suggested that while in adolescents brain activity is more driven by the emotional content of stimuli, in adults it is more attention- and goal-driven (Monk et al., 2003), although more experimental evidence is needed in this regard. From a developmental science perspective, it is fundamental to study adolescence because it is a crucial transition time for maturation and reorganization of cognitive, affective and social functions (Spear, 2000; Casey et al., 2008). Moreover, because of this developmental reorganization, adolescents, compared with adults, often exhibit greater emotional reactivity, which may put them at risk for hazardous behavior and increased vulnerability to mood and anxiety disorders Received 20 January 2009; Accepted 16 July 2009 This work is supported by National Institute of Health K23 RR18638-01, Marshall Reynolds Foundation, and University of Illinois at Chicago Magnetic Resonance Center for Research Pilot Fund. Correspondence should be addressed to Alessandra M. Passarotti, Center for Cognitive Medicine, Institute for Juvenile Research, Department of Psychiatry, University of Illinois at Chicago, 912 South Wood Street (M/C 913), Chicago, IL 60612, USA. E-mail: [email protected]. (Nelson et al., 2005; Blakemore, 2008; Casey et al., 2008). It has, in fact, been suggested that abnormal development of affect regulation functions may be intrinsic to pediatric bipolar disorder (Pavuluri et al., 2006; Pavuluri and Passarotti, 2008), anxiety disorders (Thomas et al., 2001), child depression (Silk et al., 2006) and autism (Davidson and Slagter, 2000). Therefore, it is crucial to elucidate the neurodevelopmental trajectory of the underlying brain systems in healthy child and adolescent populations, also to be able to accurately characterize the deviance from normal development of affect regulation and social behavior in such clinical populations. To this aim, Nelson et al. (2005) propose a developmental model of social information processing in terms of a neural network that has three interactive neural nodes, each with different functions and developmental timetables. A ‘detection’ node (composed of intraparietal sulcus, superior temporal sulcus and inferior temporal and occipital regions) detects socially relevant stimuli and matures in the first few years of life. An ‘affective’ node processes the emotional significance of detected social stimuli, and includes regions engaged by reward and punishment (i.e. amygdala and other limbic regions, ventral striatum, hypothalamus and in certain circumstances orbitofrontal cortex), which are primary sites of gonadal steroids and hormonal action. They also undergo functional and anatomical reorganization during puberty due to surge of gonadal hormones by regulation of other neurotransmitter systems (Giedd et al., 1996). Developmental studies suggest that the amygdala is fairly functional in processing emotions since the first few years Published by Oxford University Press. For Permissions, please email: [email protected] Downloaded from http://scan.oxfordjournals.org/ at UIC Library, Collections Development on March 24, 2015 Our knowledge on the development of the affective and cognitive circuitries that underlie affect regulation is still limited. This functional magnetic resonance imaging (fMRI) study examined whether there is more efficient prefrontal modulation of affective circuits with development. Ten adolescents (mean age 14 2 years) and 10 adults (mean age 30 6 years) underwent two scanning conditions that required different levels of cognitive control over face emotion processing. A ‘directed’ emotion processing condition required judgment of facial expressions. An ‘incidental’ emotion processing condition required an age judgment. For the incidental emotion processing condition, adolescents, compared with adults, showed less activation in right ventrolateral prefrontal cortex (VLPFC) and greater activation in paralimbic regions, suggesting greater emotional reactivity and immature prefrontal circuitries for affect regulation. For the directed emotion processing condition, adolescents, compared with adults, showed decreased recruitment of both the dorsal and pregenual right anterior cingulate cortex (ACC), suggesting immature modulatory functions of the ACC during directed face emotion processing. These results indicate that the neural circuitries for affect regulation are still developing in adolescence and have not yet reached the adult level. 388 SCAN (2009) at fearful faces, with development. Abnormal brain maturation during adolescence may affect development of these three nodes, with consequent dysregulation in their interactions that may increase vulnerability to mood and anxiety disorders in adolescence (Nelson et al., 2005). One way to test whether an increase in prefrontal regulation of sub-cortical activity is a possible bio-behavioral mechanism for the development of emotion regulation and control functions (Blumberg et al., 2004; Nelson et al., 2005; Marsh et al., 2006; Bell and Deater-Deckard, 2007; YurgelunTodd, 2007) is to compare brain activation in adolescents and adults when emotion processing is unconstrained as compared with when it is under cognitive control. Studies in healthy adults (Critchley et al., 2000; Hariri et al., 2000; Gorno-Tempini et al., 2001) and patient populations with emotional dysregulation (Chen et al., 2006; Pavuluri et al., 2009) have examined the level of engagement of corticolimbic circuitry during incidental or directed processing of emotions. In adults, a directed face emotion processing condition (which requires explicit judgment of facial emotions) tends to elicit the concerted interaction of cortical and limbic structures, with the VLPFC and ACC directly regulating amygdala activity (Gorno-Tempini et al., 2001). On the other hand, the incidental condition, which usually requires subjects to attend to non-emotional face aspects (e.g. gender or age) (Gur et al, 2002), is usually characterized by an automatic increase in amygdalar reactivity in response to the emotional content of faces (Hariri et al., 2000; Gorno-Tempini et al., 2001), while the prefrontal cortex (PFC) attends to non-emotional aspects. Therefore, this condition has been used to more selectively probe sub-cortical reactivity. Studies with healthy adults (Critchley et al., 2000; Hariri et al., 2000; Keightley et al., 2003) have reported greater amygdala activation during incidental than during directed face emotion processing, which has been explained in terms of greater automatic affective response of the amygdala when the PFC is not directly modulating emotion processing. Finally, a developmental fMRI study by Monk et al. (2003) found that during unconstrained attention, adolescents engaged limbic regions more than adults, but only adults showed differential activation of the OFC when attending to emotional as compared with non-emotional face aspects. These developmental findings are in agreement with Nelson’s model (Nelson et al., 2005) and suggest that while brain activity is relatively more emotionally driven in adolescents, with development it becomes more attentionand goal driven (Monk et al., 2003; Nelson et al., 2005). Building from previous developmental work (Monk et al., 2003), the present developmental fMRI study is one of the first to compare brain activation for incidental and directed face emotion processing in 12- to 18-year-old adolescents with that of adults, and to investigate whether improved affect regulation with age is associated with greater PFC control of activity in sub-cortical emotion circuitries. Our participants underwent two task conditions. A ‘directed’ Downloaded from http://scan.oxfordjournals.org/ at UIC Library, Collections Development on March 24, 2015 of life (Johnson, 2005), although at least one study found volumetric changes in the amygdala of 4- to 18-year-old males (Giedd et al., 1996), which may be associated with ongoing functional maturation. Moreover, these regions modulate cognitive processes by driving attention towards socially relevant stimuli. The ‘cognitive-regulatory’ node controls impulse inhibition and goal-directed behavior and consists of dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC) and orbitofrontal cortex (OFC). The VLPFC and dorsal anterior cingulate cortex (dACC) are considered to be a neural interface between cognitive and affective processes (Petrides and Pandya, 2002; Pavuluri et al., 2007; Pavuluri and Passarotti, 2008). The VLPFC is involved in inhibition processes (Kemmotzu et al., 2005), and it also modulates amygdala emotional reactivity via the rostral portion of the ACC (Petrides and Pandya, 2002; Pavuluri et al., 2007). Moreover, the VLPFC has direct connections with the DLPFC, which is involved in cognitive control, working memory and executive functions (MacDonald et al., 2000) so that interactions between VLPFC and DLPFC enable goal-directed behavior and emotion regulation. The VLPFC (Gogtay et al., 2004; Marsh et al., 2006), ACC (Conel, 1967), DLPFC (Giedd, 2004) and amygdalo-cortical connectivity (Blumberg et al., 2004) develop more slowly than regions in the other two nodes, mainly through protracted myelination, synaptic pruning, maturation and learning, throughout adolescence (Giedd et al., 1999; Sowell et al., 1999). Their maturation has been associated with improvements in executive functions (Luna et al., 2001, 2004; McGivern et al., 2002) and in cognitive modulation of emotion processing (Blakemore and Choudhury, 2006). While these three nodes always interact with each other, the nature of these interactions changes with development. While emotion circuits drive attention to socially relevant stimuli early on, the cognitive regulatory node modulates emotional circuit activity more and more with maturation (Nelson et al., 2005). Studies with primates (Goldman-Rakic, 1987), humans with brain damage (Manes et al., 2002) and healthy individuals (Iidaka et al., 2001; Hariri et al., 2000; Pavuluri et al., 2006) have confirmed that the brain systems involved in affect processing and regulation are functionally and anatomically interconnected, and that with age there is an increased cognitive control of affect functions (YurgelunTodd, 2007). Recent functional magnetic resonance imaging (fMRI) studies have found that adolescents exhibit greater amygdala activation than adults while processing affective faces (Monk et al., 2003; Guyer et al., 2008; Hare et al., 2008), although at least a few studies did not find this age difference in amygdala functioning (Pine et al., 2001; Nelson et al., 2003). Moreover, Yurgelun-Todd and Killgore (2006) found increased prefrontal activity while processing fearful faces in adolescence compared with childhood, and Killgore et al. (2001) found increased prefrontal activity and decreased amygdala activity in female subjects while looking A. M. Passarotti et al. Facial emotion processing in adolescents METHODS Subjects Our participants were healthy volunteers recruited from the University of Illinois at Chicago University of Illinois at Chicago (UIC) and from the surrounding community through word-of-mouth. They were all right-handed as assessed by the Annett Handedness Questionnaire (Annett, 1970), and had normal or corrected-to-normal vision. The WASH-U-KSADS was also administered to all participants to ensure the absence of any mental disorder. Subjects were excluded from this study if they had an estimated IQ below a score of 85. All subjects had comparable IQ scores, as estimated with the Wechsler Abbreviated Scale of Intelligence (WASI, 1999). Moreover, subjects were excluded if they had a pervasive developmental disorder, neurological condition, history of head trauma with loss of consciousness for >10 min, substance use or mood disorder, use of medication that would alter cerebral blood flow (such as medication for migraine and blood pressure) or the presence of metallic implants, retractors or braces. The study was approved by the institutional review board at UIC. According to University IRB protocols, we obtained an informed consent from all adult subjects. Adolescent participants gave their assent, and an informed consent was also obtained from at least one parent or guardian. 389 Out of the initial pool of 13 adult subjects, fMRI data from three subjects were discarded due to head motion artifacts. The remaining 10 subjects (five females) had a mean age of 30 6 years. Neuroimaging data were collected on 14 healthy adolescents, and data from four of these subjects were excluded due to excessive head motion. The remaining 10 individuals (five females) had a mean age of 14 2 years, and were matched on race and gender to the adult group. There were no significant differences in the gender (Fisher’s P ¼ 1; NS) and racial (Fisher’s P ¼ 0.65; NS) composition of the two groups. fMRI session and directed and incidental emotion processing tasks The fMRI task, which lasted for 7 min, consisted of an incidental and a directed face emotion processing condition. During the incidental face processing condition, participants judged whether the presented face was older or younger than 35 years of age. During the directed face processing condition, participants judged whether the facial affect was positive/happy or negative/angry. In both conditions, every face stimulus presented had a facial expression (i.e. angry or happy) so that face stimuli had the same perceptual and emotional information in both conditions, and only task instructions differed across conditions. Responses were by button press, with the correct response key (i.e. left or right key) counterbalanced across subjects and equally randomized across trials. We used 24 happy and 24 angry faces from a database of 200 Gur faces, with established reliability and validity (Gur et al., 2002). The level of emotionality for happy and angry faces was comparable in the two task conditions based on normative data provided by Gur et al. (2002). Half of the faces from each emotion type were older and half younger than 35 years of age. Correct responses for type of face emotion and age were based on the normative data by Gur et al. (2002). Faces from this database were also randomly selected with regard to gender, valence and race (Gur et al., 2002). Each face was presented on a black background, necks and shoulders were removed, but hair was left in the images. Three angry and three happy face trials were presented in a pseudo-random order in each block of the conditions. We used the rule that the same trial type (e.g. angry face, or face >35 years of age) could not be presented more than twice in a row. Individual faces were not repeated during the task to avoid effects of repetition and familiarity. Prior to imaging studies, participants spent 20 min in a mock scanner to acclimate them to the scanner environment. Then the scanning session started. A color high-resolution LCD projector projected visual stimuli onto a rear projection screen that was viewed via an angled double-mirror system mounted on a standard GE head coil. During the scan, a camera monitored subject’s right eye to ensure attention to visual stimuli. We adopted a standard block design to maximize signal-to-noise ratio for Downloaded from http://scan.oxfordjournals.org/ at UIC Library, Collections Development on March 24, 2015 face emotion processing condition required an explicit judgment on whether a face looks happy or angry, and an ‘incidental’ face emotion processing condition required a decision on whether the emotional face presented is younger or older than 35 years (Gur et al., 2002). Based on previous adult (Critchley et al., 2000; Hariri et al., 2000; Gorno-Tempini et al., 2001) and developmental studies (Killgore et al., 2001; Monk et al., 2003; YurgelunTodd, 2007; Guyer et al., 2008; Hare et al., 2008) and on Nelson’s model (Nelson et al., 2005), we hypothesized that: (i) for the incidental condition adolescents, compared with adults, would exhibit greater automatic sub-cortical emotional reactivity during incidental emotion processing, accompanied by reduced activity in VLPFC due to cortical and corticolimbic connection immaturity; (ii) for the directed condition adolescents, compared with adults, would exhibit higher or comparable levels of amygdala activation during voluntary processing of face emotions, and reduced engagement of regions that evaluate and regulate emotion processing, such as the VLPFC and the ACC; and (iii) while in adults cortical activity would be more modulated by cognitive goals (i.e. attend to emotions or to age), in adolescents it would be more modulated by automatic emotion processing. Therefore, we predicted that while for adults engagement of cortical regions involved in regulation and evaluation of face emotions (i.e. VLPFC, ACC) would be more prominent in the directed than in the incidental condition, the opposite pattern would be present in adolescents. SCAN (2009) 390 SCAN (2009) short imaging sessions. The task consisted of four 30-s blocks of the incidental emotion processing condition (I) and four 30-s blocks of the directed emotion processing condition (D) (Figure 1). Condition blocks were separated by a 20-s fixation block (F), with the last 3 s of this period alerting the subjects on the condition block (i.e. directed or incidental) that would be presented next. For all subjects, blocks were presented in the following order: (F) I (F) D (F) I (F) D (F) I (F) D (F) I (F) D (F). On each trial, a face portraying a happy or angry expression was presented for 5 s. There were six trials in each block, for a total of 48 trials. Since processing of face emotions is more automatic than making an age decision, participants always started with the incidental condition first, in order to avoid that they may carry over explicit face emotion processing strategies from the directed condition onto the implicit condition at the beginning. Image acquisition, processing and data analysis MRI acquisitions were performed using a 3.0 T whole body scanner (Signa, General Electric Medical System, Milwaukee, WI, USA). Functional images were acquired using gradientecho echo-planar imaging, which is sensitive to regional alterations in blood flow via blood oxygenation leveldependent (BOLD) contrast effects. Twenty-five axial slices were acquired. Parameters for functional scans were: TE ¼ 25 ms; flip angle ¼ 908; field of view ¼ 20 20 cm2; acquisition matrix ¼ 64 64; TR ¼ 2.5 s; 5 mm slice thickness, with 1 mm gap. Anatomical images were acquired in the axial plane [3D gradient recalled (SPGR), 1.5 mm thick contiguous axial slices] and were later co-registered with the functional data for each subject. FIASCO software (Functional Imaging Analysis SoftwareComputational Olio; http://www.stat.cmu.edu/fiasco/) (Eddy et al., 1996) was used to estimate and correct the functional neuroimaging data for head motion. This approach implements both 3D motion estimation and correction, and reduction of cardiac and respiratory effects in k-space. FIASCO also applies a cubic function to remove slow signal drift. It provides diagnostics for identifying images with artifacts such as high shot noise and displacement that cannot be readily corrected by motion correction algorithms (Eddy et al., 1996). We excluded from the analyses individual volumes from the time series if head displacement from the median head position in the time series was >1.5 mm, or if head rotation from the median head position was >0.58. The mean number of volumes excluded from analyses for motion artifact (adolescents: mean ¼ 64, s.d. ¼ 10.37; adults: mean ¼ 58, s.d. ¼ 22.33) did not differ significantly across groups [F(1,18) ¼ 0.61; P < 0.44]. Voxel-wise effect size (r) maps were calculated for each subject for each pair-wise condition contrast (incidental condition vs fixation, directed condition vs fixation, incidental vs directed condition), and then a Fisher z-transform was performed voxel by voxel to normalize the data (zr) (Rosenthal, 1991). AFNI software (Analysis of Functional Neuroimages) (Cox, 1996) was used to transform individual subjects’ zr-maps (effect size) and SPGR anatomical images into Talairach space using AFNI’s automated Talairach procedure (Talairach and Tournoux, 1988). Finally we re-sampled the original functional maps (3.125 3.125 6 mm grid) to an isotropic 3 3 3 mm grid. In order to examine the group differences in neural activation for the incidental and directed condition, a whole brain, voxel-wise omnibus Analysis of Variance (ANOVA) was carried out in AFNI, with the betweensubjects factor of group (adolescents, adults) and the within-subjects factor of condition (incidental vs fixation, directed vs fixation, incidental vs directed). The significant interaction of group by condition was further examined through planned t-tests, which were part of the ANOVA. To correct for multiple voxel-by-voxel comparisons, significant clusters of activation were identified using a contiguity threshold (minimum volume threshold ¼ 270 mm3; minimum clustering radius: 3.1 mm) applied to the entire brain, which maintained an experiment-wise Type I error rate of a corrected P < 0.025, based on AFNI’s AlphaSim Monte Carlo simulations (Ward, 2000) that were restricted to in-brain voxels. In addition, based on findings from the whole brain ANOVA, we performed Region of Interest (ROI) analyses so that we could: (i) perform correlation analyses to determine the relationship between fMRI activation in specific ROIs and behavioral performance (RT, accuracy) or age for the two conditions in each group; and (ii) so that we could quantify and illustrate the significant group by condition interaction for selected ROIs that showed group differences in our two conditions. Anatomical ROIs Downloaded from http://scan.oxfordjournals.org/ at UIC Library, Collections Development on March 24, 2015 Fig. 1 (A) Incidental and directed conditions block design. (B) Examples of face stimulus displays used for the incidental condition (on the left) and for the directed condition (on the right). A. M. Passarotti et al. Facial emotion processing in adolescents SCAN (2009) were defined in standard Talairach space using AFNI tools. These regions in AFNI format as well as the rationale for anatomical ROI definition are available at http://ccm .psych.uic.edu/Research/NormalBrain/ROI_rules.htm; http://ccm.psych.uic.edu/Research/ResearchProgram/ NormalBrain/ROIaffect_rules.aspx. fMRI data A significant group by condition interaction [F(1,18) ¼ 5.04, P < 0.01] was further explored with planned comparisons within the ANOVA, for which we corrected for multiple comparisons and applied cluster thresholding procedures that ensured an experiment-wise Type 1 error rate of P < 0.025 (corrected) (see Methods section). Incidental vs directed condition in each group Table 2 illustrates significant findings for the comparison between the two conditions in each group. Table 1 Response time and accuracy measures for adults and adolescents on the incidental and directed emotion processing tasks Response time (ms) Incidental condition Directed condition Accuracy (%) Incidental condition Directed condition Adults Median s.d. Adolescents Median s.d. 1568.2 354.9 1223.7 257.5 1609.8 372.8 1320.3 358.8 80 92 75 83 Adolescent group. Adolescents exhibited greater activation for the incidental than for the directed condition in right PFC regions, such as the right superior frontal gyrus, DLPFC and VLPFC, in right dorsal ACC, parahippocampal gyrus and caudate. They exhibited greater activation for the directed than the incidental condition in bilateral superior temporal gyrus and in left inferior parietal lobule. Adult group. Adults exhibited greater activation for the incidental than the directed condition in right DLPFC. They showed greater activation for the directed than the incidental condition in bilateral VLPFC and medial frontal gyrus, cingulate regions (left pregenual ACC, mid-cingulate cortex and bilateral posterior cingulate cortex) and temporoparietal regions (bilateral middle temporal gyrus and left inferior parietal lobule). Group differences for incidental vs directed condition Table 3 provides a list of significant group differences for the incidental vs directed condition. The adolescent group showed greater activation than the adult group for the incidental relative to the directed condition in right DLPFC and VLPFC, dorsal ACC, posterior cingulate and inferior parietal lobule, bilateral middle temporal gyrus and precuneus. Adolescents showed less activation than adults in no brain region. Group differences for incidental or directed condition vs fixation Table 4 provides a list of significant group differences for each condition compared with fixation. Figure 2 illustrates significant group differences in brain activation in selected ROIs, and relative bar graphs of functional activation for the significant interaction of group by condition. Incidental condition. The adolescent group showed greater activation than the adult group for the incidental condition in right DLPFC/middle frontal gyrus (Figure 2A and B), right medial PFC and mid-cingulate gyrus, left precuneus and bilateral posterior cingulate gyrus, posterior face processing regions (right fusiform gyrus, bilateral middle temporal gyrus) and bilateral parahippocampal gyrus. Adults showed greater activation than adolescents for the incidental condition in right VLPFC (Figure 2C and D). Finally, compared with adults, adolescents exhibited greater right amygdala activation (Figure 2E and F), but only with a more liberal contiguity threshold, i.e. corrected P < 0.05, than that applied to the whole-brain analysis (i.e. corrected P < 0.025). Directed condition. The adolescent group showed greater activation than the adult group for the directed condition in right fusiform gyrus. Adults showed greater activation than adolescents in right pregenual ACC, bilateral dorsal ACC (Figure 2G and H) left caudate and left middle temporal gyrus. Downloaded from http://scan.oxfordjournals.org/ at UIC Library, Collections Development on March 24, 2015 RESULTS Behavioral data Median RT and accuracy data were analyzed in separate repeated measures ANOVAs. Group (adolescents, adults) was the between-subjects factor, and Condition (Incidental, Directed) was the within-subjects factor. Median RT (for correct responses) and accuracy data for each group and each condition are summarized in Table 1. With regard to median RT, no significant effects or interactions were found. There was only a non-significant trend for condition [F(1,18) ¼ 132.9; P ¼ 0.06], in that across groups median RT was faster for the directed (1272 ms) than for the incidental face emotion processing (1589 ms) condition. The accuracy data revealed higher accuracy for the directed (87%) than for the incidental (78%) condition across groups [F(1,18) ¼ 17.07, P < 0.0007], but there were no significant effects of group [F(1,18) ¼ 0.26, P < 0.62] or group by condition [F(1,18) ¼ 2.15, P < 0.16]. Finally, the difference in accuracy between the directed and the incidental condition did not differ significantly in the two groups [F(1,18) ¼ 0.93, P < 0.35] (there was an 8% difference in accuracy between the two conditions for the adolescent group, and a 12% difference in accuracy between the two conditions for the adult group). 391 392 SCAN (2009) A. M. Passarotti et al. Table 2 Incidental vs directed condition for each group Adolescents Incidental > directed Directed > incidental t-value for peak activation BA 22 BA 22 BA 40 1512 1782 486 3159 324 378 297 810 729 270 3.08 3.50 4.29 3.90 3.22 3.04 2.88 3.88 5.23 3.15 BA BA BA BA BA BA BA BA BA BA BA BA 1188 1809 1728 270 459 1053 1647 1377 405 4914 2835 2619 4.54 3.16 3.01 2.57 3.24 4.66 3.08 5.15 3.13 5.08 4.24 4.92 Region BA 32, 56, 14 44, 38, 20 26, 32, 7 5, 21, 39 8, 20, 11 38, 49, 4 22, 7, 2 59, 43, 8 64, 28, 11 58, 46, 38 Right superior frontal gyrus Right middle frontal gyrus Right inferior frontal gyrus Right dorsal ACC Right caudate Right parahippocampal gyrus Left parahippocampal gyrus Right superior TG Left superior TG Left inferior parietal lobule BA BA BA BA 44, 38, 23 11, 50, 8 1, 56, 2 53, 29, 2 49, 23, 8 7, 29, 11 1, 13, 38 14, 52, 32 16, 61, 20 59, 52, 8 55, 64, 5 52, 34, 29 Right middle frontal gyrus Right medial frontal gyrus Left medial frontal gyrus Right inferior frontal gyrus Left inferior frontal gyrus Left pregenual ACC Left mid-CG Right posterior CG Left posterior CG Right middle TG Left middle TG Left inferior parietal lobule 10 46 47 32 46 10 10 45 45 24 24 31 31 22 37 40 Talairach coordinates and t-values for peak activation in significant clusters (P < 0.025 with contiguity threshold; minimum volume threshold ¼ 270 mm3) representing greater activation in each group for one condition relative to the other. CG, cingulate gyrus; TG, temporal gyrus. Table 3 Incidental vs directed condition for adolescents vs adults Adolescents > adults Incidental vs directed Adults > adolescents Incidental vs directed None Talairach coordinates for peak values Region BA 5, 47, 26 13, 62, 17 26, 32, 7 11, 26, 32 53, 49, 1 58, 7, 7 11, 43, 23 8, 55, 38 22, 55, 41 29, 43, 35 Right medial frontal gyrus Left medial frontal gyrus Right inferior frontal gyrus Right dorsal ACC Right middle temporal gyrus Left middle temporal gyrus Right posterior cingulate gyrus Right precuneus Left precuneus Right inferior parietal lobule BA BA BA BA BA BA BA BA BA BA None None None 9 10 47 32 21 21 23/30 7 7 31 Volume (mm3) t-value for peak activation 513 702 270 405 1215 405 1188 324 837 405 4.47 3.95 2.89 3.37 3.91 3.85 2.63 3.08 4.45 2.93 None None Talairach coordinates and t-values for peak activation in significant clusters (P < 0.025 with contiguity threshold; minimum volume threshold ¼ 270 mm3) representing greater activation in one group relative to the other for the incidental vs directed condition. Correlation between behavioral performance and functional activation for the directed and incidental conditions Based on the findings of group differences in neural activation in the whole-brain analyses, Pearson correlation analyses (two tailed) were performed for each group to further examine the relationship between profiles of behavioral performance and functional activation (i.e. volume in voxels) in functionally relevant ROIs (amygdala, parahippocampal gyrus, dorsal ACC, subgenual ACC, posterior cingulate gyrus, DLPFC, VLPFC, middle temporal gyrus, precuneus, inferior parietal lobule and caudate) for the directed and the incidental condition. Regarding the incidental condition, adults exhibited a significant positive correlation between accuracy rates and activation in the right subgenual ACC (r ¼ 0.73, P ¼ 0.02), whereas adolescents exhibited only a non-significant trend in this regard (r ¼ 0.60, P ¼ 0.07). For the directed condition, both Downloaded from http://scan.oxfordjournals.org/ at UIC Library, Collections Development on March 24, 2015 Adults Incidental > directed Directed > incidental Volume (mm3) Talairach coordinates for peak values Facial emotion processing in adolescents SCAN (2009) 393 Table 4 Adolescents vs adults for each condition vs fixation Incidental vs fixation Adolescents > adults Region BA Volume (mm3) t-value for peak activation 41, 14, 29 2, 65, 5 50, 64, 29 58, 40, 7 29, 79, 13 20, 34, 1 19, 37, 1 22, 6, 12 2, 13, 29 2, 46, 26 7, 55, 11 1, 64, 23 53, 11, 23 Right middle frontal gyrus Right medial frontal gyrus Right middle TG Left middle TG Right fusiform gyrus Right parahippocampal gyrus Left parahippocampal gyrus Right amygdala* Right mid-CG Right posterior CG Left posterior CG Left precuneus Right inferior frontal gyrus BA 9 BA10 BA 39 BA 21 BA 19 BA 27 BA 30 BA BA BA BA BA 23 31 30 31 45 783 2781 378 945 621 621 864 135 729 1080 1053 810 621 2.57 3.75 3.15 2.91 4.09 2.68 4.00 2.82 3.85 3.81 3.17 3.47 4.06 32, 79, 13 52, 64, 1 17, 1, 26 6, 5, 44 11, 38, 5 40, 34, 1 Right fusiform gyrus Left middle TG Right dorsal ACC Left dorsal ACC Right pregenual ACC Left caudate BA BA BA BA BA 19 37 24 24 32 486 810 378 270 297 297 5.12 3.28 3.00 2.45 2.74 2.66 Talairach coordinates and t-values for peak activation in significant clusters (P < 0.025 with contiguity threshold; minimum volume threshold ¼ 270 mm3) representing greater activation in one group relative to the other for each condition vs fixation. *Significant cluster at P < 0.05. adolescents (r ¼ 0.83, P ¼ 0.003) and adults (r ¼ –0.66, P ¼ 0.04) showed a significant correlation between performance (in terms of accuracy for adolescents and RT for adults) and activation in the right amygdala. Finally, we carried out Pearson’s linear regression analyses with age as a predictor, both with regard to behavioral performance (RT and accuracy), and with regard to functional activation in significant ROIs that revealed group differences in the whole-brain ANOVA (DLPFC, VLPFC, amygdala and dorsal ACC). No significant results of age as a predictor were found for either condition, possibly because of sample size limitations. sub-cortical automatic emotion processing during affective challenge (Blumberg et al., 2004; Marsh et al., 2006; Bell and Deater-Deckard, 2007; Yurgelun-Todd, 2007). Furthermore, as often found in developmental fMRI studies (Monk et al., 2003; Nelson et al., 2003; Passarotti et al., 2007), these differences in neural activation occurred in the absence of significant group differences in behavioral performance for either condition, suggesting that because of brain immaturity, adolescents may need to engage cognitive and affective neural networks differently than adults in order to reach performance levels comparable with adult ones (Nelson et al., 2003). DISCUSSION In line with our first hypothesis, the present fMRI findings showed that for the incidental face emotion processing condition vs fixation adolescents, compared with adults, exhibited increased paralimbic activation (i.e. right amygdala and bilateral parahippocampal gyrus), which was also coupled with reduced activation in right VLPFC (i.e. right inferior frontal gyrus, BA 45). Confirming our second hypothesis, for directed processing of face emotions vs fixation, adolescents, relative to adults, exhibited less activation in the right dorsal and pregenual ACC (i.e. left dorsal ACC, BA 24; right pregenual ACC, BA 32), two cingulate regions interfacing with VLPFC and DLPFC functions (MacDonald et al., 2000; Petrides and Pandya, 2002), which may indicate ACC functional immaturity during face emotion processing. These findings are consistent with the viewpoint that with development, there is increased prefrontal control of Incidental vs directed emotion processing in each group For the incidental vs the directed condition, adolescents exhibited greater activation in right DLPFC, a region involved in cognitive control, attention and working memory processes (Mesulam, 1999; MacDonald et al., 2000). This is likely due to a greater attentional effort (Compton et al., 2003) for the age judgment than for the face emotion decision in adolescents. Moreover, they exhibited greater bilateral paralimbic activation, which was accompanied by greater right VLPFC and dorsal ACC activation. Furthermore, adolescents showed greater activation for the directed than for the incidental condition only in temporal regions that process facial expressions (Haxby et al., 2002), and inferior parietal regions that are part of the visuospatial attention network (Mesulam, 1999). Therefore, this pattern of results can be interpreted as Downloaded from http://scan.oxfordjournals.org/ at UIC Library, Collections Development on March 24, 2015 Adults > adolescents Directed vs fixation Adolescents > adults Adults > Adolescents Talairach coordinates for peak values 394 SCAN (2009) A. M. Passarotti et al. Downloaded from http://scan.oxfordjournals.org/ at UIC Library, Collections Development on March 24, 2015 Fig. 2 Significant group differences in activation for whole-brain ANOVA, group by condition interaction: [F(1,18) ¼ 5.04, P < 0.01] (left side), and ROI analyses (right side) for the incidental condition vs fixation (A–F) and for the directed condition vs fixation (G and H). In the whole-brain effect size maps, red clusters of activation indicate significantly (P < 0.025, corrected) greater activation in adolescents compared with adults, and blue clusters indicate significantly (P < 0.025, corrected) reduced activation in adolescents compared with adults. (A) For the incidental condition vs fixation, the adolescent group had significantly greater activation than the adult group in right DLPFC (TLRC coordinates for peak activation: 41, 14 and 29). (B) Bar graph of activation during the incidental and directed conditions relative to fixation for the right DLPFC ROI shows greater right DLPFC activation in adolescents compared with adults for the incidental condition, in the significant group by condition interaction [F(1,18) ¼ 6.25, P < 0.02]. (C) For the incidental condition vs fixation, the adolescent group exhibited significantly reduced activation than the adult group in right VLPFC (TLRC coordinates for peak activation: 53, 11 and 23). (D) Bar graph of activation during the incidental and directed conditions relative to fixation for the right VLPFC ROI shows reduced right VLPFC activation in adolescents compared with adults for the incidental condition, in the significant group by condition interaction [F(1,18) ¼ 4.26, P < 0.05]. (E) For the incidental condition vs fixation, the adolescent group exhibited significantly greater activation than the adult group in right amygdala (TLRC coordinates for peak activation: 22, –6 and –12) (in this small region, the corrected P < 0.05). (F) Bar graph of activation during the incidental and directed conditions relative to fixation for the right amygdala ROI shows greater right amygdala activation in adolescents than adults for the incidental condition in the significant group by condition interaction [F(1,18) ¼ 4.70, P < 0.04]. (G) For the directed condition vs fixation, the adolescent group exhibited significantly reduced activation, compared with adults, in right dorsal ACC (TLRC coordinates for peak activation: 17, –1 and 26). (H) Bar graph of activation during the incidental and directed conditions relative to fixation for the right dorsal ACC ROI shows reduced right dorsal ACC activation in adolescents than adults in the directed condition in the significant group by condition interaction [F(1,18) ¼ 4.16, P < 0.05]. Error bars in bar graphs represent SE values. Note that the right side of the brain image corresponds to the left hemisphere and the left side corresponds to the right hemisphere. Facial emotion processing in adolescents Incidental vs directed emotion processing between groups When directly comparing the two groups for the incidental vs directed condition, it emerged that the adolescent group showed greater activation than the adult group in right DLPFC, cingulate cortex (dorsal ACC and posterior cingulate gyrus) and parietal regions, most likely because of greater attentional effort during the age judgment condition in the younger group (MacDonald et al., 2000). Adolescents also engaged the right VLPFC more than adults for the incidental relative to the directed condition, possibly because of a greater need to regulate excessive automatic emotion processing (Adolphs, 2002; Petrides and Pandya, 2002; Pavuluri et al., 2007). Animal (Goldman-Rakic, 1987) and human (Posner and Petersen, 1990) neurophysiological studies have identified the ACC as a part of both distributed attentional and emotion processing networks (Vogt et al., 1992; Devinsky et al., 1995; Bush et al., 2000). The dorsal ACC has connections to the PFC and parietal cortex and plays an important role in conflict monitoring (Bush et al., 2000; Botvinick et al., 1999) and cognitive regulation (Posner and Rothbart, 2000; Marsh et al., 2006). The ACC undergoes protracted anatomical (Casey et al., 1997; Cunningham et al., 2002) and functional (Adelman et al., 2002; Nelson et al., 2003; Velanova et al., 2008) maturation from infancy to adolescence. Our findings of developmental differences in 395 ACC involvement for the incidental vs directed condition are similar to previous findings of differential ACC involvement in adolescents relative to adults during incidental encoding (Monk et al., 2003; Nelson et al., 2003) and passive viewing of emotional faces (Monk et al., 2003). The developmental differences in ACC engagement for the incidental vs the directed condition may be related to the fact that emotional stimuli draw attention to a greater degree in the younger age group, because there is greater emotional modulation of cognitive processing in children compared with adults (Nelson et al., 2003, 2005). Similarly, Deeley et al. (2008) found decreased dorsomedial and middle frontal cortex activation with age in response to emotional faces in healthy males, which was attributed to a possible reduction in attentional demands, or in processing the selfrelevance of facial expressions during social interactions in adulthood. Incidental face emotion processing in adolescents compared with adults When comparing the two groups for the incidental condition relative to fixation, adolescents exhibited greater activation than adults in bilateral parahippocampal regions, right amygdala (though with a less stringent probability threshold) and right fusiform gyrus, and reduced activation in right VLPFC. Increased amygdala reactivity in adolescents compared with adults was found during incidental processing of face affect (Monk et al., 2003; Guyer et al., 2008) and during an emotional go-nogo task with emotional faces (Hare et al., 2008). Moreover, the VLPFC is a region that comes to play a primary role in self-regulatory control (Marsh et al., 2006) and emotion regulation (Pavuluri et al., 2009) with development. It, therefore, seems that in adolescents, relative to adults, there is greater automatic sub-cortical reactivity to emotional content, even when emotion processing is not necessary, which is accompanied by poor VLPFC control of the emotional circuits. These findings provide some support to the hypothesis that with normal development, there is increased cortical cognitive control over automatic sub-cortical emotional reactivity, possibly resulting in better executive functions and emotional regulation (Adolphs, 2002; Marsh et al., 2006). It is interesting that recent developmental fMRI studies with adolescents suffering from affect disorders indicate a greater exacerbation of sub-cortical reactivity and reduced prefrontal regulation, and/or a delay in the normal developmental trajectory of cortical regulation of affect in these individuals. In fact, Pavuluri et al. (2009) found similar results of greater amygdala reactivity and reduced VLPFC control for incidental processing of face emotions in adolescents with bipolar disorder compared with healthy adolescents in the same age range as our participants. Furthermore, Hare et al. (2008) found that during an emotional go-nogo task with affective faces, individuals with higher trait anxiety, relative to healthy peers, did not exhibit habituation in Downloaded from http://scan.oxfordjournals.org/ at UIC Library, Collections Development on March 24, 2015 suggesting that brain activation in adolescents is relatively more modulated by the emotional content of stimuli than by task instructions, and that during the incidental condition they automatically process face affect, even when it is not task-related (see also Monk et al., 2003). Moreover, they engage ventral and medial PFC regions most likely in an attempt to regulate and evaluate automatic emotional reactivity in sub-cortical circuits. In agreement with previous findings with adults (Hariri et al., 2000; Gorno-Tempini et al., 2001) in the present study, adults showed greater activation for the directed than the incidental condition in ventral and medial prefrontal regions that typically process, evaluate and regulate emotional content (i.e. VLPFC, medial PFC and pregenual ACC) (Adolphs, 2002; Petrides and Pandya, 2002; Pavuluri et al., 2007). Similar to adolescents, adults showed greater activation in DLPFC for the incidental than the directed condition, likely due to greater attentional effort for the age judgment than the face emotion judgment (MacDonald et al., 2000; Compton et al., 2003). Therefore, confirming our third hypothesis, our data suggest that in adults, as expected, it is the directed face emotion processing condition, which requires explicit face emotion processing, that elicits greater cortical regulation of emotion processing as compared with the incidental condition, for which emotion processing is not necessary (Hariri et al., 2000; Gorno-Tempini et al., 2001; Monk et al., 2003). SCAN (2009) 396 SCAN (2009) Directed face emotion processing in adolescents compared with adults For the directed condition, adolescents exhibited greater activation than adults in right fusiform gyrus and less activation than adults in ACC regions (i.e. right pregenual ACC and bilateral dorsal ACC). The two groups did not differ significantly for amygdala activation during the directed condition. The dorsal ACC is important for cognitive conflict modulation (Bush et al., 2000; Botvinick et al., 1999), whereas the subgenual and pregenual ACC, together with the VLPFC, exert control over amygdala emotional reactivity (Bush et al., 2000; Botvinick et al., 1999). Increases in ACC volume (Casey et al., 1997) and functional activation (Velanova et al., 2008) from childhood to late adolescence have been associated with better control of cognitive conflict and error monitoring. The present findings of reduced dorsal and pregenual ACC activation in adolescents compared with adults during the condition explicitly requiring face emotion processing suggest that even in late adolescence, the cognitive and emotion modulation functions of the dorsal and pregenual ACC, respectively, are still developing. Therefore, adolescents, compared with adults, seem less able to engage in cognitive regulation of directed face emotion processing. Our findings agree with those of Williams et al. (2006) who found greater selective control of medial prefrontal systems over emotion functions in adults compared with adolescents. Lastly, both for the directed and the incidental conditions we found greater right fusiform activation in adolescents compared with adults. Greater fusiform gyrus activation in children and adolescents compared with adults has been found for face processing in previous developmental fMRI studies and has been attributed to immature specialization of regional brain function (Passarotti et al, 2003, 2007; Gathers et al., 2004; Aylward et al., 2005). Alternatively, it may be attributed to increased attentional modulation of fusiform activity by means of emotional stimuli in adolescents than in adults (Veuilleumier et al., 2001; Nelson et al., 2005). Developmental differences in the neural correlates of behavioral performance for the incidental and directed condition With regard to the directed condition, for both groups a significant positive correlation of performance measures and functional activation was found only for the right amygdala. Therefore, the right amygdala, which is crucial for processing of facial emotions (Adolphs, 2002), seems to be the closest neural correlate of directed face emotion processing performance in this study. This finding is in line with evidence of early functional specialization of the amygdala (Johnson, 2005) and with our whole-brain analyses, which did not reveal age differences in amygdala activation for the directed condition. On the contrary, our results suggest that the neural correlates of performance for the incidental condition may present a slower developmental timetable. In fact, adults showed a positive correlation between accuracy rates and activation in the subgenual portion of the right ACC, an important region that helps the VLPFC to regulate affect processes (Whalen et al., 1998; Bush et al., 2000; Kemmotzu et al., 2005; Pavuluri et al., 2007); however, adolescents showed only as a non-significant trend for this regions, which may be indicative of greater individual variability associated with ongoing functional development (Conel, 1967; Adelman et al., 2002). Finally, the present study has limitations in sample size and represented age ranges that call for future investigations with larger samples of children and adolescents across the age span. Moreover, since we adopted a block design, for which each block contained both positive and negative facial emotion trials, we were not able to contrast group differences in activation for positive vs negative facial emotions, and we also could not differentiate between neural activation for correct and incorrect responses. Therefore, future event-related fMRI studies will be useful in differentiating neural mechanisms that underscore processing of negative vs positive emotional content as well as correct relative to incorrect responses for directed vs incidental processing of emotions. CONCLUSIONS The present fMRI findings provide initial evidence that progressive cortical control over sub-cortical emotional circuits may represent the normal developmental trajectory of emotion regulation systems, and that this process continues through adolescence. Future investigations with larger samples and greater age ranges will help define the normative developmental timetable of maturation in the neural bases of emotional self-regulation. These investigations could also potentially inform clinical models on the possible neural deviations from the normal developmental trajectory of emotion regulation circuitries that may lead to affect dysregulation in developmental populations (Blumberg et al., 2004; Nelson et al., 2005; Bell and Deater-Deckard, 2007; Pavuluri and Passarotti, 2008). Downloaded from http://scan.oxfordjournals.org/ at UIC Library, Collections Development on March 24, 2015 amygdala reactivity over repeated stimulus exposure, and this was also associated with reduced functional connectivity between VLPFC and amygdala. Finally, it is of interest that adolescents with anxiety disorder, compared with healthy controls, were found to exhibit amygdalar hyper activation (McClure et al., 2007; Beesdo et al., 2009) and increased VLPFC and ACC activity (McClure et al., 2007) in response to fearful faces when focusing their attention on internally experienced fear (i.e. during subjective-state monitoring of fear). These findings point to the importance of further studying how emotional circuits may abnormally shape the cognitive-attentional systems during development in children and adolescents with mood disorders (Nelson et al., 2005). A. M. Passarotti et al. Facial emotion processing in adolescents Conflict of Interest Dr A.M.P. has no financial relationships to disclose. Dr J.A.S., unrelated to this work, has received support from NIMH, GlaxoSmithKline, AstraZeneca, Janssen and Eli Lilly. Dr M.N.P’s work, also unrelated to this manuscript, is supported by NARSAD Independent Investigator Award, NICHD, NIMH, Dana Foundation, American Foundation for Suicide Prevention, GlaxoSmithKline-NeuroHealth (Investigator-initiated study), Astra Zeneca (Speaker), Abbott Pharmaceuticals (Study Drug) and Janssen Research Foundation (Study Drug). Annett, M. (1970). A classification of hand preference by association analysis. British Journal of Psychology, 61, 303–21. Adelman, N.E., Menon, V., Blasey, C.M., et al. (2002). A developmental fMRI study of the stroop color-word task. Neuroimage, 16, 61–75. Adolphs, R. (2002). Neural systems for recognizing emotion. Current Opinions in Neurobiology, 12, 169–77. Aylward, E.H., Park, J.E., Field, K.M., et al. (2005). Brain activation during face perception: evidence of a developmental change. Journal of Cognitive Neuroscience, 17(2), 308–19. Bell, M.A., Deater-Deckard, K. (2007). Biological systems and the development of self-regulation: integrating behavior, genetics and psychophysiology. Journal of Developmental and Behavioral Pediatrics, 28(5), 409–20. Beesdo, K., Lau, J.Y.F., Guyer, A.E., et al. (2009). Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Archives of General Psychiatry, 66(3), 275–85. Blakemore, S.J., Choudhury, S. (2006). Development of the adolescent brain: implications for executive function and social cognition. Journal of Child Psychology and Psychiatry, 47, 296–312. Blakemore, S.J. (2008). The social brain in adolescence. Nature Neuroscience, 9, 267–77. Blumberg, H.P., Kaufman, J., Martin, A., Charney, D.S., Krystal, J.H., Peterson, B.S. (2004). Significant of adolescent neurodevelopment for the neural circuitry of bipolar disorder. Annals of New York Academy of Sciences, 1021, 376–83. Botvinick, M., Nystrom, L.E., Fissell, K., Carter, C.S., Cohen, J.D. (1999). Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature, 402, 179–81. Bush, G., Luu, P., Posner, M.I. (2000). Cognitive and emotional influences in anterior cingulated cortex. Trends in Cognitive Sciences, 4(6), 215–22. Casey, B.J., Trainor, R., Giedd, J., et al. (1997). The role of the anterior cingulate in automatic and controlled processes: a developmental neuroanatomical study. Developmental Psychobiology, 30, 61–9. Casey, B.J., Jones, R.M., Hare, T. (2008). The adolescent brain. Annals of New York Academy of Sciences, 1124, 111–26. Compton, R.J., Banich, M.T., Mohanty, A., et al. (2003). Paying attention to emotion: an fMRI investigation of cognitive and emotional stroop task. Cognitive Affective and Behavioral Neuroscience, 3, 81–96. Chen, C.H., Lennox, B., Jacob, R., et al. (2006). Explicit and implicit facial affect recognition in manic and depressed states of bipolar disorder: a functional magnetic resonance imaging study. Biological Psychiatry, 59, 31–9. Conel, J.L. (1967). The Postnatal Development of the Human Cerebral Cortex, (Vols I–VIII). Harvard: Harvard University Press. Cox, R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computational Biomedical Research, 29, 162–73. Critchley, H., Daly, E., Phillips, M., et al. (2000). Explicit and implicit neural mechanisms for processing of social information from facial 397 expressions: a functional magnetic resonance imaging study. Human Brain Mapping, 9, 93–105. Cunningham, M.G., Bhattachraryya, S., Benes, F.M. (2002). Amygdalocortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. The Journal of Comparative Neurology, 453, 116–30. Davidson, R.J., Slagter, H.A. (2000). Probing emotion in the developing brain. Mental Retardation and Developmental Disabilities, 6, 166–170. Deeley, Q., Daly, E. M., Azuma, R., et al. (2008). Changes in male brain responses to emotional faces from adolescence to middle age. NeuroImage, 40, 389–97. Devinsky, O., Morrell, M.J., Vogt, B.A. (1995). Contributions of anterior cingulate cortex to behavior. Brain, 118, 279–306. Eddy, W.F., Fitzgerald, M., Genovese, C.R., Mockus, A., Noll, D.C. (1996). Functional image analysis softwarecomputational olio. Proceedings in Computational Statistics, Part A. Heidelberg: Physica, pp. 39–49. Gathers, A.D., Bhatt, R., Corbly, C.R., Farley, A.B., Joseph, J.E. (2004). Developmental shifts in cortical loci for face and object recognition. NeuroReport, 15, 1549–53. Giedd, J.N., et al. (1996). Quantitative MRI of the temporal lobe, amygdale and hippocampus in normal human development: ages 4–18 years. Journal of Comparative Neurology, 366, 223–30. Giedd, J.N., Blumenthal, J., Jeffries, N.O., et al. (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience, 2, 753–8. Giedd, J.N. (2004). Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences, 1021, 77–85. Goldman-Rakic, P.S. (1987). Development of cortical circuitry and cognitive function. Child Development, 58, 601–22. Gogtay, N., Giedd, JN, Lusk, L., et al. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Science USA, 101(21), 8174–9. Gorno-Tempini, M.L., Pradelli, S., Serafini, M., et al. (2001). Explicit and incidental facial expression processing: an fMRI study. NeuroImage, 14, 465–73. Gur, R.C., Sara, R., Hagendoorn, M., et al. (2002). A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. Journal of Neuroscience Methods, 115, 137–43. Guyer, A.E., Monk, C.S., Mcclure-Tone, E.B., et al. (2008). A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience, 20, 1565–82. Hare, T.A., Tottenham, N., Galvan, A., Voss, H.U., Glover, G.H., Casey, B.J. (2008). Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry, 63, 927–34. Hariri, A.R., Bookheimer, S.Y., Mazziotta, J.C. (2000). Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport, 17, 43–8. Haxby, J.V., Hoffman, E.A., Gobbini, M.I. (2002). Human neural systems for face recognition and social communication. Biological Psychiatry, 51(1), 59–67. Iidaka, T., Omori, M., Murata, T., et al. (2001). Neural interaction of the amygdala with the prefrontal and temporal cortices in the processing of facial expressions as revealed by fMRI. Journal of Cognitive Neuroscience, 13(8), 103. Johnson, M. (2005). Subcortical face processing. Nature, 6, 766–74. Keightley, M.L., Winocur, G., Graham, S.J., Mayberg, H.S., Hevenor, S.J., Grady, C.L. (2003). An fMRI study investigating cognitive modulation of brain regions associated with emotional processing of visual stimuli. Neuropsychologia, 41, 585–96. Kemmotzu, N., Villalobos, M.E., Gaffrey, M.S., Courchesne, E., Muller, R.A. (2005). Activity and functional connectivity of inferior frontal cortex associated with response conflict. Cognitive Brain Research, 24, 335–42. Killgore, W.D., Oki, M., Yurgelun-Todd, D.A. (2001). Sex-specific developmental changes in amygdala responses to affective faces. Neuroxreport, 12, 427–33. Downloaded from http://scan.oxfordjournals.org/ at UIC Library, Collections Development on March 24, 2015 REFERENCES SCAN (2009) 398 SCAN (2009) Petrides, M., Pandya, D.N. (2002). Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and cortico-cortical connection patterns in the monkey. European Journal of Neuroscience, 16, 291–310. Pine, D.S., Grun, J., Zarahn, E., et al. (2001). Cortical brain regions engaged by masked emotional faces in adolescents and adults: an fMRI study. Emotion, 1, 137–47. Posner, M.I., Petersen, S.E. (1990). The attention systems if the human brain. Annual Reviews of Neuroscience, 13, 25–42. Posner, M.I., Rothbart, M.K. (2000). Developing mechanisms of selfregulation. Developmental Psychopathology, 12, 427–41. Rosenthal, R. (1991). Meta-analytic Procedures for Social Research. Newbury Park, CA: Sage. Silk, J.S., Shaw, D.S., Skuban, E.M., Oland, A., Kovacs, M. (2006). Emotion regulation strategies in offspring of child-hood onset depressed mothers. Journal of Child Psychology and Psychiatry, 47(1), 69–78. Sowell, E.R., Thompson, P.M., Holmes, C.J., Jernigan, T.L., Toga, A.W. (1999). In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience, 2, 859–61. Spear, L.P. (2000). The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Review, 24, 417–63. Talairach, J., Tournoux, P. (1988). Co-planar Stereotactic Atlas of the Human Brain. Stuttgart, New York: Thieme Medical Publishers. Thomas, K.M., Drevets, W.C., Dahl, R.E., et al. (2001). Amygdala response to fearful faces in anxious and depressed children. Archives of General Psychiatry, 58, 1057–63. Velanova, K., Wheeler, M.E., Luna, B. (2008). Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral Cortex, 18(11), 2505–22. Veuilleumier, P., Armony, J.N., Driver, J., Dolan, R.J. (2001). Effects of emotion and attention on face processing in the human brain: an event-related fMRI study. Neuron, 30, 829–41. Vogt, B.A., Finch, D.M., Olson, C.R. (1992). Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cerebral Cortex, 2, 435–43. Whalen, P.J., Rauch, S.L., Etcoff, N.L., McInerney, S.C., Lee, M.B., Jenike, M.A. (1998). Masked presentation of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience, 18, 411–18. Ward, B. (2000). ALPHASIM, National Institute of Health, Bethesda. http:// afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf. Wechsler Abbreviated Scale of Intelligence (WASI) (1999). Psychological Corporation. San Antonio, TX: Harcourt Brace & Company. Williams, L.M., Brown, K.J., Palmer, D., et al. (2006). The mellow years?: neural basis of improving emotional stability over age. The Journal of Neuroscience, 26, 6422–30. Yurgelun-Todd, D. (2007). Emotional and cognitive changes during adolescence. Current Opinions in Neurobiology, 17(2), 251–7. Yurgelun-Todd, D.A., Killgore, W.D. (2006). Fear-related activity in the prefrontal cortex increases with age during adolescence: a preliminary fMRI study. Neuroscience Letters, 406, 194–9. Downloaded from http://scan.oxfordjournals.org/ at UIC Library, Collections Development on March 24, 2015 Luna, B., Thulborn, K.R., Munoz, D.P., et al. (2001). Maturation of widely distributed brain function subserves cognitive development. NeuroImage, 13, 786–93. Luna, B., Garver, K.E., Urban, T.A., Lazar, N.A., Sweeney, J.A. (2004). Maturation of cognitive processes from late childhood to adulthood. Child Development, 75, 1357–72. MacDonald, A.W., Cohen, J.D., Stenger, V.A., Carter, C.S. (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science, 288, 1835–8. Manes, F., Sahakian, B., Clark, L., et al. (2002). Decision-making processes following damage to the prefrontal cortex. Brain, 125(Pt 3), 624–39. Marsh, R., Zhu, H, Schultz, R.T., et al. (2006). A developmental fMRI study of self-regulatory control. Human Brain Mapping, 27, 848–63. Mcclure, E.B., Monk, C.S., Nelson, E.E., et al. (2007). Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry, 64(1), 97–106. McGivern, R.F., Andersen, J., Byrd, D., Mutter, K.L., Reilly, J. (2002). Cognitive efficiency on a match to sample task decreases at the onset of puberty in children. Brain and Cognition, 50, 73–89. Mesulam, M.M. (1999). Spatial attention and neglect: parietal, frontal and cingulated contributions to the mental representation and attentional targeting of salient extrapersonal events. Philosophical Transactions of the Royal Society, B, 354, 1325–46. Monk, C.S., Mclure, E.B., Nelson, E.E., et al. (2003). Adolescent immaturity in attention-related brain engagement to emotional facial expressions. NeuroImage, 20, 420–8. Nelson, E.E., Mcclure, E.B., Monk, C.S., et al. (2003). Developmental differences in neuronal engagement during implicit encoding of emotional faces: an event-related fMRI study. Journal of Child Psychology and Psychiatry, 44, 1015–24. Nelson, E.E., Leibenluft, E., Mcclure, E.B., Pine, D.S. (2005). The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine, 35, 163–74. Passarotti, A.M., Paul, B.M., Bussiere, J., Buxton, R., Wong, E., Stiles, J. (2003). Development of face and location processing: a fMRI study. Developmental Science, 6(1), 100–17. Passarotti, A.M., Smith, J., DeLano, M., Huang, J. (2007). Developmental differences in the neural bases of the face inversion effect show progressive tuning of face-selective regions to the upright orientation. NeuroImage, 34(4), 1708–22. Pavuluri, M.N., Shenkel, L.S., Aryal, S., et al. (2006). Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. American Journal of Psychiatry, 163(2), 286–93. Pavuluri, M.N., O’Connor, M.M., Harral, E.M., Sweeney, J.A. (2007). Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biological Psychiatry, 62, 158–67. Pavuluri, M.N., Passarotti, A.M. (2008). Neural bases of emotional processing in pediatric bipolar disorder. Expert Review of Neurotherapeutics, 8(9), 1381–7. Pavuluri, M.N., Passarotti, A.M., Harral, E.M., Sweeney, J.A. (2009). An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 48(3), 308–18. A. M. Passarotti et al.