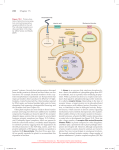
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download the scf ubiquitin ligase: insights into a molecular machine
P-type ATPase wikipedia , lookup
Cell growth wikipedia , lookup
Spindle checkpoint wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Histone acetylation and deacetylation wikipedia , lookup
Magnesium transporter wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Phosphorylation wikipedia , lookup
Cytokinesis wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein structure prediction wikipedia , lookup
Signal transduction wikipedia , lookup
Protein moonlighting wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Protein domain wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Proteolysis wikipedia , lookup
REVIEWS THE SCF UBIQUITIN LIGASE: INSIGHTS INTO A MOLECULAR MACHINE Timothy Cardozo and Michele Pagano Abstract | Ubiquitin ligases are well suited to regulate molecular networks that operate on a posttranslational timescale. The F-box family of proteins — which are the substrate-recognition components of the Skp1–Cul1–F-box-protein (SCF) ubiquitin ligase — are important players in many mammalian functions. Here we explore a unifying and structurally detailed view of SCF-mediated proteolytic control of cellular processes that has been revealed by recent studies. CYCLIN-DEPENDENT KINASE (CDK). A protein kinase that controls cell-cycle progression in all eukaryotes and requires physical association with cyclins to achieve full enzymatic activity. F-BOX PROTEIN (FBP). A component of the machinery for the ubiquitindependent degradation of proteins. F-box proteins recognize specific substrates and, with the help of other subunits of the E3 ubiquitin ligase, deliver them to the E2 ubiquitin-conjugating enzyme. Department of Pathology and New York University Cancer Institute, New York University Medical Center, 550 First Avenue, MSB 599, New York, New York 10016 USA. Correspondence to M.P. e-mail: michele.pagano@ med.nyu.edu doi:10.1038/nrm1471 Fundamental cellular functions such as DNA replication, mitosis, DNA repair, transcription, cell differentiation and cell death are accomplished by large, multi-protein and precisely regulated molecular machines. Under most biological conditions, the driving forces of these machines — for example, the CYCLIN-DEPENDENT KINASES (CDKS) in the eukaryotic cell cycle — need to be humming constantly. Crucial modular components must therefore lock into place or detach and disappear in an instant to keep the machines from spinning out of synchronization with the rest of the cell. The cellular timescale of these active machines does not lend itself to the appearance or disappearance of ratelimiting components by the processes of transcription or translation. Instead, the disappearance of a component in a sudden and compartment-restricted manner can be a finely tuneable brake (if the target component is a catalyst) or a sensitive accelerator (if the target component is a brake or an inhibitory subunit). This type of regulated disappearance can be achieved by the ubiquitin–proteasome system (BOX 1). Recent findings have enhanced our appreciation of this system, and here we will review our current understanding of an important ubiquitin ligase — the SCF ligase — with a particular emphasis on the mammalian enzymes. The SCF ligase relies on one of its components — the F-BOX PROTEIN (FBP) — for its specificity, and the result is that the F-box family of proteins has emerged as the effector class of proteins for a crucial, NATURE REVIEWS | MOLECUL AR CELL BIOLOGY and therapeutically exploitable, step in the ubiquitinmediated degradation of many cell-cycle-regulatory proteins and transcription factors. The SCF ubiquitin ligase and the FBP family E3 ubiquitin ligases (BOX 1) have been classified into three groups: the single-subunit RING-FINGER type, the multisubunit RING-finger type and the HECT-domain type. Most of the multi-subunit RING-finger type of E3 ligases contain a cullin protein (Cul1–5, Cul7 and so on; so named because they appeared to ‘cull’, or sort, substrates for degradation)1. Many structural and functional details have now been deciphered for the most well-characterized mammalian cullin-dependent ligase (CDL) — the SCF (SKP1–CUL1–FBP) LIGASE. In this ligase, the cullin subunit Cul1 functions as a molecular scaffold that simultaneously interacts at the amino terminus with the crucial adaptor subunit Skp1 (S-phase-kinase-associated protein-1) and at the carboxyl terminus with a RING-finger protein (Rbx1, which is also known as Roc1, or Roc2) and a specific E2 enzyme or UBIQUITIN-CONJUGATING ENZYME (UBC), such as Ubc3, Ubc4 or Ubc5. Skp1, in turn, binds to one of many FBPs. Each FBP appears to be matched with a discrete number of specific substrates through a protein–protein interaction domain. The F-box domain. The FBPs are defined by the presence within their sequence of the Skp1-binding F-box domain. The first F-box gene — CCNF, which VOLUME 5 | SEPTEMBER 2004 | 7 3 9 ©2004 Nature Publishing Group REVIEWS Box 1 | The ubiquitin–proteasome system The cellular abundance of many key brakes or accelerators of cellular proliferation — a large number of which are known to be oncoproteins or tumour suppressors — is controlled by the ubiquitin–proteasome degradation system in a compartment-specific and unidirectional manner108,55. The small soluble protein ubiquitin is transferred and attached to these target substrate proteins by the sequential action of E1, E2 and E3 enzymes109,110. The E1 enzyme — of which there is only one in humans — activates the entire cellular pool of ubiquitin molecules. E2 enzymes, or ubiquitin-conjugating enzymes (UBCs), inherit the activated thiol-ester-bonded ubiquitin from the E1 enzyme. The specificity of the ubiquitylation is then provided by the E3 ligase enzyme, which binds both the target substrate and the activated ubiquitin–E2 complex, and ultimately completes the transfer of ubiquitin to the target. Several rounds of ubiquitin conjugation can produce long chains of ubiquitin moieties (polyubiquitylation), the first of which is covalently bound to the substrate. At this point, the polyubiquitylated substrate is committed to association with, and unfolding and degradation by, the 26S proteasome111, whereas monoubiquitylated proteins have non-proteolyic fates112. RING-FINGER A protein-sequence motif corresponding to a particular folded protein domain that binds Zn2+ through a four-point arrangement of cysteine and histidine amino acids. In the E3 ubiquitin ligases, this domain seems to be responsible for binding the E2 ubiquitinconjugating enzymes. SCF UBIQUITIN LIGASE A multisubunit ubiquitin ligase that contains Skp1, a member of the cullin family (Cul1), and an F-box protein, as well as a RINGfinger-containing protein (Roc1/Rbx1). UBIQUITIN-CONJUGATING ENZYME (UBC). An enzyme (also known as E2) that accepts ubiquitin from a ubiquitin-activating enzyme (E1) and, together with a ubiquitin ligase (E3), transfers it to a substrate protein. β-PROPELLER A compact structural domain, or protein-folding pattern, in which similarly sized β-sheets are stacked and offset into a complete cylinder, so that they resemble the blades of a propeller. 740 encodes cyclin F — was discovered fortuitously in a search for candidate genes at the polycystic kidney disease (PCKD) gene locus2. Although apparently unrelated to PCKD, the gene was notable for the presence of a cyclin domain. Cyclin-F levels were later shown to oscillate during the cell cycle to a similar extent as those of cyclins A and B (REF. 3), but with a distinct peak in G2 and a trough in mitosis prior to that of cyclin B (REF. 4). Most importantly, cyclin F was found to be a suppressor of the yeast Cdc4 mutant that displays G1–S deficiency and an inability to degrade Sic1, an inhibitor of Cdk1. A search for other suppressors of the Cdc4 mutation led to the discovery of yeast Skp1, the inactivation of which resulted in cell-cycle arrest in both G1 (prior to S-phase entry) and G2 (prior to mitosis). These arrests also coincided with the stabilization of key CDK regulators, and Skp1 was shown to be required for the ubiquitinmediated proteolysis of these proteins. Skp1 was able to bind to cyclin F, to Cdc4 and to many other proteins through a conserved 40-amino-acid domain. Since it was originally noted in cyclin F, this domain became known as the F-box and the F-box family of proteins was born5. Cyclin F and Cdc4 were therefore the first F-box proteins to be identified. A year later, the different pieces of the puzzle came together when it was shown that Cul1, Skp1 and Cdc4 form an E3 ligase that is required for Sic1 ubiquitylation, and this complex was called the SCF ubiquitin ligase6,7. Mammalian F-box-protein classification. The mammalian FBPs have been named according to the structural class of their substrate-binding domains 8,9 (TABLE 1), although some original names have been conserved for historical reasons. The substrate-binding domain of the FBP almost invariably lies directly carboxy-terminal to the F-box domain in the sequence, and no FBP seems to have more than one F-box domain (according to the Pfam database; see the online links box). One class of FBPs are known as FBWs (‘FB’ for F-box and ‘W’ for WD-40 repeat domain); their substrate-binding domain is a β-PROPELLER structure that is | SEPTEMBER 2004 | VOLUME 5 found in many protein-binding contexts10 and seems to recognize specific Ser/Thr phosphorylation (pS/pT) consensus sequences: DpSGXXX(X)pS (where ‘X’ represents any amino acid) in Fbw1 (also known as β-Trcp1); and a variable L[I/L/P][pS/pT]P sequence in Fbw7 and Cdc4. In the case of the FBLs (‘L’ for LEUCINE-RICH REPEAT (LRR)), the domain is an arc-shaped α–β-repeat structure that is also found in many protein-binding contexts, including the extracellular-binding domain of certain surface receptors11,12. In most cases, FBLs also seem to involve substrate phosphorylation for their interaction, but this does not seem to be a general phenomenon in LRR-containing proteins. In addition, some substrates are required to be part of specific protein complexes to be recognized by the specific FBLs and, at least in one case, recognition also requires an accessory subunit (see below). The third class of FBPs includes proteins that are known as FBXs, and that do not have WD-40 repeats or LRRs but often have different protein–protein interaction domains such as CASH (carbohydrate-interacting), cyclin box, CH (calponin homology), TDL (Trafdomain-like), Sec7, zinc-finger and proline-rich domains. Human FBXs have been identified that are specific for N-glycan moieties13,14, and the FBP-related Von-Hippel–Landau protein (VHL), which is a subunit of a CDL2 E3 ligase, has a proclivity for a hydroxylated HIF1α substrate. This indicates that, in addition to phosphorylation and glycosylation, the mode of binding of FBPs to substrates could be through a wide range of post-translational modifications such as methylation, ribosylation, farnesylation and acetylation. If it is true that FBPs are specific for these modifications, the implication is that SCF ligases are constructed to indirectly sense the initial target-modifying activity — the kinase, glycosylase, acetylase, ribosylase and so on — which would be consistent with the view of SCFs functioning as a brake and an accelerator of a molecular machine that is driven by these activities. F-box-protein functions The FBPs Skp2, β-Trcp and Fbw7 target known substrates that implicate them in the control of cellular proliferation (see below). The substrate for Fbx5, which is also known as Emi1 (early mitotic inhibitor-1) is not known. But, this protein is also central to cell proliferation because of its ability to inhibit, through means other than targeted degradation, the critical M–G1 ubiquitin ligase — the ANAPHASE-PROMOTING COMPLEX/CYCLOSOME (APC/C) — during the S and G2 phases of the cell cycle15,16. Evolutionary data (BOX 2) and data on the functions of several other human FBPs however, indicate that they might be involved in a wide variety of organspecific functions (TABLE 1). Mutations in Fbw4 (also known as dactylin in the mouse) or its orthologues (such as Hagoromo in zebrafish) result in embryonic patterning defects that manifest as limb and digit dysplasia in the mouse and stripe-pattern disorganization in the fish17,18. The corresponding human congenital malformation is known as www.nature.com/reviews/molcellbio ©2004 Nature Publishing Group REVIEWS Table 1 | Overview of mammalian F-box proteins and their known functions Mammalian F-box protein (FBP) name* (number of FBPs) Aliases Human approved gene symbol‡ Main substrates Comments Fbw1 β-Trcp1 BTRC Emi1, Cdc25a, Wee1, β-catenin, IκB-family members Gene-knockout phenotype: defective spermatogenesis, subtle mitotic defects, centrosome overduplication Fbw4 Dactylin SHFM3 Unknown Human split hand–foot malformation (SHFM) gene FBXW6 Unknown In addition to being part of an SCF ligase, it also forms a complex with Skp1 and Cul7; the latter interacts with SV-40 large T antigen Cdc4, Sel10 FBXW7 Cyclin E, Myc, Jun, Notch-1 and -4 Gene-knockout phenotype: embryonic lethal at E11, probably due to morphogenetic cardiovascular defects. Mutations in ovarian and breast cancer cell lines Skp2 SKP2 p27, p21, p57, p130 Gene-knockout phenotype: hypoplasia in most organs; endoreduplication; centrosome overduplication; defect of mitotic entry. Overexpressed in human tumours Cyclin F CCNF Unknown Function unknown. First-identified mammalian FBP FBXO2 Unknown Recognizes N-glycans. ER-associated degradation FBXO5 Unknown Inhibitor of APC/C. Overexpressed in breast tumours FBXO6 Unknown Recognizes N-glycans FBXO32 Unknown Involved in skeletal muscle atrophy. Higher expression in muscle cells FBWs (17) Fbw6 Fbw7 FBLs (22) Fbl1 FBXs (39) Fbx1 Fbx2 Fbx5 Emi1 Fbx6 Fbx32 Mafbx, Atrogin1 *The nomenclature is based on that used in references 8–10. ‡The human approved gene symbol is that recommended by the HUGO Gene Nomenclature Committee (HGNC). APC/C, anaphase-promoting complex/cyclosome; Cdc, cell division cycle; Cul7, cullin-7; E11, embryonic day 11; Emi1, early mitotic inhibitor-1; ER, endoplasmic reticulum; IκB, inhibitor of nuclear factor (NF)κB; FBL, F-box and leucine-rich-repeat protein; FBW, F-box and WD40-domain protein; FBX, F-box-only protein; SV40, simian virus-40. LEUCINE-RICH REPEAT (LRR). A protein-sequence motif that contains regular occurrences of the amino acid Leu, which are present as tandem arrays in certain proteins. The back-toback set of motifs was found to correspond to a small subdomain structure in the protein that stacks next to adjacent repeats to form a parallel, β-sheet, arc-like structure. ANAPHASE-PROMOTING COMPLEX/CYCLOSOME (APC/C). Anaphase is the phase of mitosis during which condensed chromosomes separate into sister chromatids and move along the mitotic spindles to opposite poles of the cell. The APC/C is a multisubunit E3 ubiquitin ligase with at least two alternative forms, which are activated by two different proteins (Cdc20 or Cdh1) and are necessary for the transition into anaphase, as well as the exit from mitosis and the maintenance of the G1 state. split hand–foot malformation19,20. Fbw6 shows an apparent interchangeability of an SCF key interface (see below) in that this FBP assembles an SCF ligase with either Cul1 or Cul7 (REF. 21). Cul7 was found to bind the simian virus 40 large T antigen, and its targeted disruption in the mouse resulted in abnormal vascular development22. Interestingly, another component of this CDL7 complex — Fap68 or glomulin — is the gene product that is associated with human familial venous malformations. CDL7Fbw6 therefore seems to have a role in vascular morphogenesis. Data on other FBPs are still preliminary. Fbx2 and Fbx6 are part of SCF ligases that recognize N-glycan moieties on endoplasmic-reticulum proteins that are translocated to the cytoplasm for degradation13,14. Expression of Fbx3 is increased in activated or proliferating joint epithelium (synovium) from patients with rheumatoid arthritis23. A heat-shock protein, α-crystallin, which is implicated in the pathogenesis of desmin-related myopathy, interacts with Fbx4 in a manner that is dependent on the mitotic-specific phosphorylation of certain serines in α-crystallin24. This indicates that α-crystallin might be a substrate or a cofactor of Fbx4. Fbx32 (also known as Mafbx or Atrogin1) is the product of one of only two genes that are upregulated in a panel of rat models of muscle atrophy25–27. Deficiency of this protein conferred resistance to muscle atrophy and the gene is induced by the Foxo family of transcription factors. NATURE REVIEWS | MOLECUL AR CELL BIOLOGY How SCF ubiquitin ligases modulate CDKs Despite the expected diversity of substrate targets, multi-subunit E3 ubiquitin ligases are all thought to use a similar mechanism, with the diversity of their molecular-machine substrates determining the diversity of their cellular functions. The prototype of the mechanistic signature for the E3 ubiquitin ligases is the SPINDLE CHECKPOINT in mitosis, in which the onset of anaphase is arrested globally in the cell by a signal that emanates from the KINETOCHORE28. Elegant studies in yeast showed that Mad and Bub, the key proteins that control the spindle checkpoint, function by sequestering Cdc20, a key activator of APC/C29,30. Once it is released by the appropriate signal, which indicates that spindles have achieved the appropriate tension and that metaphase chromosomes have lined up in the proper fashion, APC/C induces the ubiquitin-mediated proteolytic degradation of securin, a key inhibitor of sister-chromatid separation. Such a compartment-restricted ‘hairtrigger’ point cannot rely on transcription or translation for its execution, and eukaryotic biology has evolved an elegant solution to this need: at the prescribed time on the post-translational timescale, a readily activatable E3 ubiquitin ligase (the APC/C) degrades a key component (securin) that is functioning as a brake for its target molecular machine . Mitosis and the DNA-synthesis phase (S phase) require exceptional fidelity and precision to correctly manage the massive DNA-replication and CDC20 VOLUME 5 | SEPTEMBER 2004 | 7 4 1 ©2004 Nature Publishing Group REVIEWS Box 2 | Genomics and evolution Saccharomyces cerevisiae (~11) Fungi (~50) Caenorhabditis briggsae/elegans (~75/350) Nematoda (~425) Metazoa (~600) Fruitfly (~30) Arthropoda (~50) Chordata (~300) Mouse (73) Human (78) Eukaryota (~1,600) Viruses (~20) Archaea (~0) Bacteria (~1) Cyanobacteria (~0) Synechocystis PCC (~0) Rice spp. (~300) Arabidopsis thaliana (~500–600) Green plants (~1,000) Plastid group (~1,000) Other eukaryotes (<10) The distribution of genes that encode F-box proteins (FBPs) in several genomes is evident from genomic analysis (see figure), with several mammalian FBPs having important orthologues in lower organisms113. The figure shows the taxonomic distribution of FBPs from the InterPro database (see the online links box). Outer circles represent distal branches of the evolutionary tree (that is, genus and species; as opposed to the inner circles, which represent order and kingdom). The true number of FBPs for any organism is consistently less than that found in InterPro. There are actually 78 FBPs in humans and 73 in mice as opposed to the higher number that can be found in InterPro, and the other species numbers have been interpolated accordingly. The evolutionary distribution of the FBPs mirrors the mechanistic role of the interchangeable FBP in the otherwise invariant SCF (Skp1–Cul1–FBP) ubiquitin ligase. For any given species, there are very few Cul1 (cullin-1) and Skp1 (S-phase-kinase-associated protein-1) proteins and a correspondingly larger number of FBPs (1 Skp1 and 78 FBPs in humans versus 10–20 Skp1 proteins and 350 FBPs in Caenorhabditis elegans114,115, for example). Individual plant cells and their resident compartment-restricted molecular machines might be more transparently positioned to respond immediately to important environmental signals such as light116. Indeed, plants — such as Arabidopsis thaliana with its 500–600 genes that encode FBPs and 20 Skp1-like genes117,118 — show a comparatively larger increase in the number of FBPs over metazoans. Interestingly, although it was previously thought that no true FBPs existed in viruses or bacteria, several are now present in the InterPro and Pfam databases (see the online links box). This indicates a picture of FBP restriction to higher eukaryotic functions with rare adaptation by bacteria via gene transfer, and similarly rare pathogenic exploitation by viruses. SPINDLE CHECKPOINT The molecular process that specifically controls the assembly of the kinetochore on the chromosomal centromere and the timing of kinetochore dissociation. Dissociation involves the movement of the kinetochores, along with their attached sister chromatids, to opposite poles of the mitotic spindle during anaphase. KINETOCHORE The complicated protein assembly that links the specialized areas of condensed chromosomes that are known as centromeres to the microtubulebased mitotic spindle. 742 chromosome-segregation machines. In addition, these two important molecular machines of the cell cycle are necessarily interrelated — there is a clear need for inhibition of the synthetic machinery while mitosis is occurring, and vice versa. Finally, the behaviour of complex systems such as that of the cell cycle provides a fundamental paradox: a cyclic process has difficulty in producing checkpoints, and a system that produces checkpoints has trouble in maintaining repetitive cycles31. Clearly, the biologically diverse contexts of the cell cycle in higher eukaryotes (for example, embryogenesis, organ renewal and wound healing) require the ability to engage both endless repetitive cycles and checkpoints to ensure the synchronization of the cycle with surrounding cellular and tissue-based processes. A perspective that combines what we know about the activity of CDKs in the cell cycle and what we now know about SCF-complex function hints at how the cell resolves this paradox. G1 phase. p21 and p27 are key inhibitors of both Cdk1 (REFS 32,33) and Cdk2 (REF. 33). Seen through the prism of CDK-activity profiles in the cell cycle (BOX 3), the finding | SEPTEMBER 2004 | VOLUME 5 that SCFSkp2 is responsible for the ubiquitin-mediated degradation of p27 (REFS 34–38) and p21 (REFS 39,40) during the G1–S checkpoint reveals the role of this SCF ligase. First, it executes the transition to S-phase by degrading these CDK inhibitors; and second, it maintains Cdk1 and Cdk2 activities by keeping the environment clear of p21 and p27 later in the cell cycle (BOX 3 figure, parts a and b). Interestingly, the other main cell-cycle E3 ubiquitin ligase — APC/C — completes the picture.APC/CCdh1 maintains the attenuation of both Cdk1 and Cdk2 during G1 by simultaneously targeting Skp2 (REFS 41,42) and its cofactor Cks1 for degradation, and continuing its degradation of cyclins A and B, which was initiated during mitosis. Cyclin A binds to both Cdk2 and Cdk1, and cyclin B activates only Cdk1. The first action of APC/CCdh1 therefore preserves the inhibition of Cdk1 and Cdk2 by p27 and p21, and the second keeps the levels of cyclins A and B low during G1. Additionally, APC/CCdh1 degrades the key Cdk1-activating phosphatase Cdc25a during this period43. The conditional inactivation of the APC/C subunit Apc2 in quiescent hepatocytes causes unscheduled cell-cycle re-entry, possibly by these convergent mechanisms44. G1–S, S and G2 phases. A switch in the regulation of Cdk1 and Cdk2 that involves two other FBPs occurs during the G1–S transition and in the S and G2 phases (BOX 3 figure, part b). First, the FBP Emi1 accumulates as a result of transcription that is induced by the transcription factor E2F(REF. 15; which also induces transcription of the genes that encode cyclins A, B and E). The accumulated Emi1 inhibits APC/CCdh1 by a non-proteolytic mechanism45. Interestingly, this simultaneously releases Skp2 and cyclins A and B from APC/CCdh1-mediated destabilization. This begs the question: how is Cdk1 attenuated when its activating cyclin subunits are both stabilized and transcribed? This is where the second FBP comes in. For its full activity, Cdk1 requires the activity of the Cdc25a phosphatase that counteracts an inactivating phosphorylation by the kinase Wee1. SCFβ-Trcp has recently been shown to degrade phosphorylated Cdc25a in the S and G2 phases46,47. So, a balanced picture emerges that explains the maintenance of Cdk2 activity and Cdk1 attenuation. The activities of Cdk2–cyclin-E and Cdk2–cyclin-A are maintained by the action of SCFSkp2 on p21 and p27 and that of Emi1 on APC/CCdh1; and low Cdk1 activity is maintained by SCFβ-Trcp, despite the loss of cyclin-A and -B destabilization by APC/CCdh1 and p21 and p27 degradation through SCFSkp2. Cdk2 also helps in inactivating APC/CCdh1 by phosphorylating Cdh1 to inhibit its binding to APC/C30. This places Emi1 and Cdk2 both upstream and downstream of APC/CCdh1, which indicates that some unknown factor (X? in BOX 3 figure, part b) initiates a loop during this period of the cell cycle. Alternatively, Emi1 and Cdk2 might seem to be both upstream and downstream of APC/C if they are part of a constitutive feedback loop, only one arm of which (for example, only the inhibition of Cdh1 by Cdk2, and not the destabilization of cyclins A and B by Cdh1) is active enough to be apparent under experimental conditions. The ‘quiet’ arm of the loop www.nature.com/reviews/molcellbio ©2004 Nature Publishing Group REVIEWS Box 3 | CDK activity profiles a G1 phase b G1–S, S and G2 phase p27 APC/CCdh1 Emi1 Cyclin E Cdk1 and Cdk2 SCFSkp2 p27 p21 APC/CCdh1 X? Cyclins A and B Cdc25a Cdk2 SCFSkp2 Cdk1 p21 Cyclin A Cdk1 Cyclin B SCFβ-Trcp d Mitotic progression and exit SCFFbw7 SCFSkp2 Securin APC/CCdc20 Cyclins A and B Cyclin E Cdk2 p27 Separase Emi1 Wee1 c G2–M phase Cohesins SCFβ-Trcp Cdc25a Emi1 Cdk1 APC/CCdh1 Cyclins A and B SCFβ-Trcp Cdc25a p21 Cdk1 Wee1 Cyclin-dependent kinases (CDKs) represent both the engine and the pacemaker of the cell cycle. It is now clear that proteolysis is a significant force that imposes the required checks and balances on this engine. During G1, Cdk1 and Cdk2 need to be idle to avoid premature DNA synthesis and mitosis (see figure, panel a). Starting at the G1–S checkpoint — the point at which DNA replication and centrosome duplication begins — Cdk2 markedly increases its activity (see figure, panel b). The activity of Cdk2, and therefore the phosphorylation of its substrates, builds to a peak through the DNA-synthesis and centrosome-duplication phase into G2, at which point the activity of Cdk2 is attenuated again. The coincidence of peaking Cdk2 activity and peaking activity of the DNA-replication and centrosome-duplication machinery indicates that Cdk2 somehow releases and maintains the nuclear environment that is necessary for the synthetic machinery to function. Although Cdk2 seems to be the optimal agent for this purpose, it is not absolutely required. Low Cdk1 activity and/or the action of other CDKs contribute to these processes, and can accomplish them in the absence of Cdk2, at least under normal cellular conditions3. At around the same time that Cdk2 activity attenuates in G2, Cdk1 markedly increases its activity, building the unique mitotic cellular environment of its phosphorylated downstream substrates (see figure, panel c). Anaphase is sharply demarcated by a sudden decline of Cdk1 activity, and both Cdk1 and Cdk2 are maintained in their inactive states until the next G1–S transition (see figure, panel d). See main text for more details. X? represents an unknown factor (see main text). Blue boxes signify activated forms of the respective proteins. APC/C, anaphase-promoting complex/cyclosome; Cdc25a, cell-division cycle-25a; Emi1, early mitotic inhibitor-1; SCF, Skp1–Cul1–F-box-protein; Skp, S-phase-kinaseassociated protein. might emerge periodically in vivo without the need for an initiating factor. APC/CCDC20 According to the convention for multi-subunit E3 ligases, the presumed activator, or substratetargeting, subunit is shown in superscript text. So, the E3 ligase that is formed by the APC/C subunits and Cdc20 is written as shown, and the SCF ligase formed by Skp1, Cul1, Roc1 and the F-box protein Skp2 is known as SCFSkp2. G2–M transition. Interpreting the states of CDK activity at the G2–M transition through the E3 ligases that control them is similarly revealing (BOX 3 figure, part c). Cdk2 attenuation is now maintained by SCFFbw7-mediated degradation of its necessary partner cyclin E (REFS 48–50), although some Cdk2 activity probably remains thanks to the presence of cyclin A. Cdk1 activity is now maintained by the continued inhibition of APC/CCdh1, as well as APC/CCdc20, by Emi1; and by the renewed availability of Cdc25a because SCFβ-Trcp actively degrades it only in S and G2 phase. SCFβ-Trcp continues to have a role in this phase, however, through its NATURE REVIEWS | MOLECUL AR CELL BIOLOGY induced degradation of the Cdk1 inhibitor Wee1 (REF. 51). Notably, this is also the point at which Tome1 — an antagonist of Wee1 that contains an F-box-like domain — appears, further promoting Cdk1 activity52. Mitosis. In late mitosis, both CDKs are attenuated. The block on APC/C is removed by yet another activity of SCFβ-Trcp — the ubiquitin-mediated degradation of Emi1 (REFS 53,54). APC/CCdc20 is therefore released and, in addition to its anaphase-promoting induction of the degradation of securin, actively induces the degradation of cyclins A and B, thereby quenching Cdk1 activity (BOX 3 figure, part d). Interestingly, Cdk1 activates APC/CCdc20 by phosphorylating several APC/C subunits, thereby contributing to its own attenuation30. VOLUME 5 | SEPTEMBER 2004 | 7 4 3 ©2004 Nature Publishing Group REVIEWS This model emphasizes the contribution of E3 ligases to the regulation of CDKs. There are, of course, crucial contributions by CDK transcription, translation and translocation mechanisms (for reviews, see REFS 3,55), which were omitted to focus on how the SCF complexes work together with the cascade of kinases that execute the cell cycle. The phosphorylation sites through which the SCF complexes are primed and targeted are frequently created by kinases — both CDKs and nonCDKs — which are themselves indirectly affected by the resulting degradation in a feedback loop. The requirement of SCF for the phosphorylation of its substrates indicates that part of how these E3 ligases contribute to the execution of these checkpoints is to sense the activity of these kinases indirectly. For example, p27 ubiquitylation by SCFSkp2, which ultimately results in the activation of Cdk1 and Cdk2, requires the CDK-dependent phosphorylation of its carboxyl terminus56–58. Cyclin E is targeted for ubiquitylation by SCFFbw7 through its phosphorylation at several sites by Cdk2 and glycogensynthase kinase 3β (GSK3β)48–50,59. Cdc25a is primed through its phosphorylation by Chk1, and its β-Trcpbinding motif is phosphorylated by an as-yet-unidentified kinase46,47. The Wee1 kinase is phosphorylated by a Polo-like kinase-1 (Plk1) after priming by Cdk1-dependent phosphorylation, this forms an auto-amplification loop that connects these three central mitotic kinases (Wee1, Plk1 and Cdk1)51. Similarly, phosphorylation by Plk1 and Cdk1 targets Emi1 to β-Trcp60. In summary, Skp2 is an activator of both Cdk1 and Cdk2; Fbw7 is an inhibitor of Cdk2; and β-Trcp contributes by turning Cdk1 off during the S and G2 phases, turning it on at the G2–M transition, and finally turning it off again at the end of mitosis. These three FBPs also regulate important transcription factors: E2F1 and Myc are regulated by Skp2; Myc, Jun, and Notch-1 and -4 are regulated by Fbw7; and β-catenin and nuclear factor κB (NFκB) are regulated by β-Trcp1. In turn, these transcription factors, among several other functions, control CDK activities by inducing or suppressing the transcription of genes that encode certain CDK subunits and their regulators. Notably, SCF ligases and APC/C control each other: in G1, APC/C Cdh1 induces the degradation of Skp2 and Cks1, thereby maintaining the G1 state; and in early mitosis SCFβ-Trcp activates APC/CCdc20 by inducing the degradation of Emi1. So, a picture emerges of waves of different SCF ligases that instantaneously modify components of the CDK regulatory network to maintain, activate or attenuate these drivers of the cell cycle, apparently for the purpose of establishing checkpoints in an endless cycle. Structural picture of the SCF ubiquitin ligase ALLOSTERIC SITE A site on an enzyme, which, on binding of a modulator, causes the protein to undergo a conformational change that might alter the catalytic or binding properties of the enzyme. 744 Why is the SCF ligase constructed the way it is? The mechanism of most known protein enzymes involves the specific binding of a protein to a chemical substrate such that the substrate is positioned and orientated relative to a key catalytic atom or residue. The current collection of structural information on the SCF ligases indicates a similar mechanism, but, interestingly, this | SEPTEMBER 2004 | VOLUME 5 mechanism seems to operate at the macromolecular level of protein subunits rather than residues and secondary-structure elements. So, instead of the transfer of a small chemical group (for example, a phosphate group in a kinase), an entire protein (ubiquitin) is transferred. Instead of a key catalytic residue, as in common acid–base catalysis, there is an activated protein subunit — the activated E2 enzyme. Instead of a substratebinding subdomain, there is an entire specific substrate-binding protein (the FBP). And, instead of the orientation of the donor and acceptor groups, as well as ALLOSTERIC shifts, being encoded by the interrelationships of a single folded protein chain, these are encoded in the SCF ligase by the structural characteristics of the cullin protein and the FBP and by their respective protein surfaces and interfaces. Alternative mechanisms are also possible. These include the possibility that SCF might have oligomeric forms, that modifications occur at the cullin protein, and that the binding of certain SCF factors to both the amino and carboxyl termini of the cullin protein regulate its activity (reviewed in REF. 61). This latter observation, in particular, indicates that the mechanism of ubiquitin ligation proceeds through a significant cooperative structural change in the ligase from one end to the other. Other possibilities are that substrate unfolding or SCF oligomerization are required to bring the substrate and ubiquitin together — perhaps also with other unknown intermediate molecules — for ligation to occur. However, as structural information is available only for the SCF complex and not for possible cooperating factors, we will explore here the model that depicts the SCF ligase as a kind of ‘super-enzyme’ with an active-site-like ‘hot zone’ and briefly discuss alternative models thereafter. Crystal structures have been published of: the SCFSkp2(F-box only) complex (which contains only the F-box domain of Skp2), the complete Skp1–Skp2 complex, the Skp1–β-Trcp complex, the Skp1–Cdc4 complex, and related CDL and E2-enzyme complexes62–68. The common structures are remarkably consistent; they enable reliable three-dimensional models of complete SCF ligases to be built from the different structures, and allow the differences between them to be interpreted with respect to their mechanism of action. The models show that different protein subunits fit together into a single rigid C-shaped superstructure with a distance of around 59 Å between the E2 enzyme on one end and the substrate-binding surface of the FBP on the other. The mechanistic model that is indicated by the structures is that the 59-Å space — into which the substrate might float due to its affinity for the FBP-binding domain — is presumed to be a kind of hot zone, or super-sized active site, within which a ubiquitin molecule is likely to attach as soon as an appropriate acceptor atom is in reach. This is not unlike classic enzyme catalysis except on a larger molecular scale. This hot zone is encoded by the identities, stoichiometry and features of the SCF subunits. Interestingly, this space is reasonably well conserved in the structurally divergent single-subunit HECT ubiquitin ligases69,70, which indicates that www.nature.com/reviews/molcellbio ©2004 Nature Publishing Group REVIEWS Aa Ab B Helix 8 Skp2 VHL 'Safety belt' Ac Ad Helix 7 Homology Elongin C Skp1 Elongin B Figure 1 | The interface between the F-box domain of Skp2 and Skp1. Aa | Backbone ribbon depiction of the F-box domain of Skp2 (S-phase-kinase-associated protein-2) with consensus conserved residues highlighted: hydrophobic residues (yellow); Pro residue (red); positively-charged residues (green). Ab | Electrostatic surface of the Skp1-binding face of the F-box domain of Skp2 in the same orientation as in Aa, pictured through a skeletal ribbon representation of the Skp1 molecule. Ac | Electrostatic surface of the F-box-binding face in Skp1 with a skeletal ribbon representation of the F-box domain from Skp2. This view is rotated 180o around the vertical axis with respect to Aa and Ab. Ad | A ‘docked’ picture of the two electrostatic surfaces of Skp2 and Skp1 in the same orientation as in Ac. B | Comparison of the equivalent interfaces of the F-box domain of Skp2 with Skp1 and the cyclindependent-ligase-2–Von-Hippel–Landau-protein (CDL2VHL) complex. There is a structural homology between elongin C and the central domain of Skp1 (see boxed areas). However, helices 7 and 8 in Skp1 contribute to the orientation of the F-box domain and its attached protein-binding domain with Skp2. By contrast, no interaction is seen in this area (indicated by a dashed line) in the CDL2VHL complex. The segment of Skp2 that is referred to as a ‘safety belt’ might pin down bound substrates into the concave surface of the leucine-rich repeat. Part B of the figure is modified with permission from REF. 64 © (2002) Macmillan Magazines Ltd. many E3 ligases might use the same mechanism, but that the localized properties and critical interfaces in the super-enzyme can sometimes be accomplished within a single protein chain. Of all the localized properties that are indicated by these structures, one of the most important is the rigidity of the central portion of the superstructure, as it has the purpose of correctly positioning the donor and acceptor entities in space. The central portion of the SCF ligase was shown to consist primarily of the cullin protein, Skp1, the F-box domain and possibly the intervening segment (linker) between the F-box domain and the substrate-binding (WD-40, LRR and so on) domain of the FBP. The introduction of flexible linkers that eliminate the rigidity of the Cul1 scaffold inactivates SCFSkp2 without affecting its ability to bind the substrate or the E2 enzyme67. Likewise, mutations that were designed to affect the rigid coupling between the F-box domain and substrate-binding domains in Cdc4 disrupted its function in vivo71. What is seen in the structure of the SCF ligase might apply to other E3 ligases such as CDL2VHL. VHL residues that are important for the relative arrangement, and probably also the rigidity, of its α and β domains — which bind the elongin-C adaptor and the Hif1-α substrate, respectively — are frequently mutated in the VHL cancerpredisposition syndrome65. Also, mutations in Cbl, NATURE REVIEWS | MOLECUL AR CELL BIOLOGY another E3 ligase, that affect the rigid linkage between the E2-binding and substrate-binding domains abolished its function without significantly affecting its ability to bind either substrate or E2 (REF. 72). Because there are only a few E2-ubiquitin donors and many target substrates, another critical protein interface is found at the transition from the conserved E2-binding site on one side of the rigid central portion, to the diversified substrate-binding FBP on the other side. In SCF, this transition point is between Skp1 and the F-box domain. The F-box domain is therefore like an anchor that can bring a wide variety of substrates to the SCF ligase. Accordingly, the structural manifestations of the distinctive F-box-domain sequence patterns are nonspecific folding and proteinsurface features (FIG. 1Aa). The F-box domain has a compact trihelical conformation that forms an interlocked heterodimer with a broad, shallow pocket in Skp1. The structure shows that the strictly conserved portions of the sequence motif correspond to key folding features. This includes the signature Pro residue, which precisely breaks the first helix of the domain, and the only conserved charged residue — a Lys — that seems to enforce the turn between helices 2 and 3. Other key residues form a conserved hydrophobic surface that is continuous with the folding core of the domain and that binds to Skp1 (FIG. 1Ab). VOLUME 5 | SEPTEMBER 2004 | 7 4 5 ©2004 Nature Publishing Group REVIEWS The remainder of the F-box-domain surface is electronegative to ensure that the correct orientation will be achieved by repulsion of the largely electronegative surface that surrounds the hydrophobic target pocket of Skp1. This produces the tight interlocked complex (FIG. 1Ac,Ad). Even slight disturbances in the orientation of this key transition point might be amplified at the distal protein-binding surface of the FBP — with functional consequences. For example, mutation of a Skp1 residue that is important for the precise arrangement of the Skp1–Skp2 interface was shown to disrupt Skp1 function in yeast without abolishing Skp1–Skp2 binding 64. Although the core F-box-domain–Skp1 interaction is similar in the crystal structures of Skp1–Skp2, Skp1–β-Trcp and Skp1–Cdc4, a carboxy-terminal βstrand of Skp2 seems to rigidify the spatial position of the LRR in Skp2 by participating in the core F-boxdomain–Skp1 complex, whereas the same region of Skp1–Cdc4 and Skp1–β-Trcp comprises only Skp1 and F-box-domain atoms. Remote elements in the components of different SCF complexes, such as the carboxyl terminus in Skp2, might therefore contribute different pieces to this core interface structure. The cullin component of the complex might also contribute, and, indeed, Cul1 and probably Cul7 (REF. 22) are implicated in this interface. Again, this theme might be a general one in the E3-ligase family, as a subdomain of Skp1 and elongin C of CDL2VHL are structurally similar. The α-domain of VHL is structurally equivalent to the FBP and interacts with elongin C in the same orientation as the FBP does with Skp1 (FIG. 1b). When the structures of these two complexes are compared, however, equivalent portions of Skp1 are missing. These include Skp1 helices 7 and 8, which are notably less conserved in Skp1 homologues, and might be provided by other proteins such as the cullins, or might not be necessary. Finally, in the recently described CDL3, the equivalent of the Skp1–FBP interface is entirely within a single polypeptide chain73–76. So, although the ‘jigsaw puzzle’ of this interface is constructed in many different ways, these might all just be different solutions to the common problem of rigidly positioning the distal substrate in the hot zone, at least in the structurally supported model of SCF function. Versatile F-box proteins DEGRON A protein element, usually a sequence motif, that targets the protein for proteolytic degradation. 746 Has nature designed FBPs to be flexible enough on their substrate-binding end to bind biophysically diverse substrates — for example, those intercalated into complex molecular machines — while concurrently achieving a convergence to the common jigsaw-puzzle interface with Skp1? The FBP recognition sites of SCFβ-Trcp and SCFCdc4 consist of phosphorylated protein loops (seen as peptides in the crystal structures) that tether substrates, such as β-catenin and cyclin E, that have high affinity for the WD-40 domains of their respective SCFs. β-catenin is a globular soluble oncoprotein that is phosphorylated on its FBP-recognition site (‘DEGRON’) by GSK3β77–80. The spacing between this degron tether and the key ubiquitin-accepting Lys residue is stringent and seems to be a mechanistic determinant for some β-Trcp substrates | SEPTEMBER 2004 | VOLUME 5 such as β-catenin and IκB66 (inhibitor of NFκB). Furthermore, this type of substrate–FBP interaction seems characteristic for WD-40 domains, with the key FBW residues and the substrate peptide clustering at the top of the narrow channel in the centre of the cylindrical β-Trcp WD-domain. The Skp1–F-box-domain interaction is similar in both SCFs. So, according to the model whereby the substrate is stably tethered to the WD-40 domain, an acceptor Lys is present in a predictable location in space and the F-box domain is appropriately docked in Skp1, all that seems to be required to position the acceptor Lys is a relatively simple, rigid, linker region. This consists primarily of a rigid helix, between the F-box domain and the WD-40 domain (FIG. 2a). Other β-Trcp substrates, such as Cdc25a, which contains a variant phospho-degron, show that the structural arrangement of their FBP-recognition sites and ubiquitin-acceptor groups is not overly stringent. Therefore, various SCF substrates, which might have different, but closely related, arrangements of these sites might bind FBPs that are similarly constructed (that is, FBPs that comprise a rigid helix that links the F-box domain and the substrate-binding domain). Indeed, a yeast substrate of SCFCdc4 — Sic1 — is a very different type of substrate compared with β-catenin and cyclin E: it is a disordered protein with several lowaffinity sites for interaction with, and ubiquitylation by, its FBP. The most efficient ubiquitylation sites of Sic1 are on its amino terminus where several acceptor Lys residues are found. Mutation of these Lys residues abolishes polyubiquitylation of Sic1 if it is in complex with Cdk1, but not if it is free81. The implication is that Cdc4, although restrained by the fact that it has only one binding site for free Sic1, can position a ubiquitinacceptor Lys from one of the many different binding sites on free Sic1 into the correct spatial region. Free Sic1 might ‘dance’ on the single Sic1-binding site on Cdc4 — thereby bringing potential acceptor Lys residues into the hot zone, and possibly increasing the likelihood of this occurrence with each ubiquitin addition82. When in complex with Cdk1, Sic1 might be less structurally flexible and, therefore, more easily positioned through a particular phosphorylation site by the SCF ligase, although substantial segments might still be disordered. It remains to be seen whether the first of these intriguing observations — the regulated degradation of a free disordered protein — occurs in the cell. But this versatility might be a signature feature of the SCF-ligase mechanism that is indicated by these structures: all the substrates simply require the positioning of acceptor Lys residues in the hot zone, and the FBP has evolved to achieve this task. PHOSPHORECOGNITION seems to function in a fundamentally different manner in FBLs compared with the FBWs, and might vary from FBL to FBL as required by the substrate. The mode of FBL–substrate binding is not yet known — there are two FBLs with known substrates (human Skp2 and yeast Grr1), but only Skp2 has been crystallographically resolved. Several three-dimensional structures of non-FBP LRRs have been determined www.nature.com/reviews/molcellbio ©2004 Nature Publishing Group REVIEWS a UBC β-Trcp 59 Å Ub Substrateinteraction domain Linker Rbx1 F-box domain Skp1 Cul1 b Cdk2 p27 UBC 59 Å Cks1 Ub Skp2 Rbx1 Cul1 Skp1 Figure 2 | Models of the complete SCF-ligase complexes with their substrate targets. a | A model of the Skp1–Cul1–F-box-protein (SCF)β-Trcp complex bound to the phosphorylated destruction-box peptide of β-catenin, which is shown in atom-coloured, space-filling depiction (carbon, grey; nitrogen, blue; oxygen, red). Each protein subunit is labelled by name in the same colour as its stucture. The location of the ~59-Å space between the predicted ubiquitin site and the F-box substrate-binding interface (the ‘hot zone’) is indicated. Adapted from REF. 66. b | Equivalent view of SCFSkp2 next to a model of the Cks1–Cdk2–p27 complex (where Cks1 stands for Cdc28-protein-kinase regulatory subunit-1 and Cdk2 stands for cyclin-dependent kinase-2), which was produced by superimposition of the CDK domains from the Cks1–Cdk2 structure and the p27–Cdk2–cyclin-A structure. In the underlying crystallographic structure, p27 is a fragment of the protein amino-terminal to residue Thr187. A dashed red line indicates how the backbone up to residue Thr187 might extend in the complex to the Cks1–Skp2 bimolecular interface, possibly forming a circular complex. Adapted from REF. 67. Cul1, cullin-1; Rbx1, RING-box protein-1; Skp, S-phase-kinase-associated protein; Ub, ubiquitin; UBC, ubiquitinconjugating enzyme (or E2 ubiquitin ligase). PHOSPHORECOGNITION The process by which a specific binding site on one protein recognizes a specifically constructed site on another protein that has been posttranslationally modified by the addition of a phosphate group to amenable side chains such as Ser or Thr. experimentally, however, and many of them contain their ligands in the complex, which bind in the concave surface of the LRR arc11,12. If enough repeats are present, LRRs might form an almost complete circle that encloses a small protein ligand. The substrates of yeast Grr1, Cln1 and Cln2 are multi-phosphorylated in a non-sequence-specific manner for their recognition83, and the phospho-interaction surface of yeast Grr1 was predicted to be in this concave surface where a patch of mutation-sensitive cationic residues lie84. NATURE REVIEWS | MOLECUL AR CELL BIOLOGY The prototype human FBL, Skp2, also requires phosphorylation of at least one of its substrates on a specific residue that is part of a CDK phosphorylationconsensus motif (residue Thr187 in p27). The interaction of Skp2 with phosphorylated p27 also requires the association of Skp2 with Cks1 (REFS 85,86). The binding site on SCF that binds Thr187-phosphorylated p27 is thought to be a bimolecular site that is formed by Skp2 and Cks1 (REF. 87). This is consistent with the fact that either Cks1 or Skp2 alone bind Thr187-phosphorylated p27 poorly — as both proteins normally participate in the interaction. An Asp residue in Skp2 (see space-filling group in FIG. 2b) that is critical for the interaction of Skp2 with Cks1 is located at the edge of the concave surface of Skp2 (REF. 88). Taken together, these data indicate the possibility that at least one portion of p27 and/or Cks1 binds in the LRR concave surface of Skp2, as shown in the known structures of LRR–ligand complexes. The interaction between the phosphorylated Thr187 residue of p27 and Cks1 probably occurs at that same LRR concave site, but these proteins might also interact at another site (that is, there are two binding determinants). Indeed, unphosphorylated p27 was shown to bind to Skp2 with low affinity 89. The loosely packed carboxy-terminal strand that is seen in the concave LRR surface of the Skp2 structure (FIG. 1b) might therefore serve as a kind of molecular ‘safety belt’ for the substrate-binding determinants: the amino terminus of this strand originates from the LRR tip and its carboxyl terminus is locked back into the jigsaw-puzzle interface of the F-box domain. So, if Skp2 represents FBLs in general, post-translationally modified substrates might bind in relatively diverse ways, and the binding might be rigidly coupled to the Skp1–F-box-domain interface, through features like the safety-belt loop, just as in the FBWs. Studies on p27 degradation by Skp2 showed that it must be complexed to a CDK to undergo Skp2-mediated degradation. Three well-established Skp2 substrates — p27, p21 and p57 — are thought to be unstructured, and partly disordered, free proteins that become ordered on association with their target complex90–92. At least in the case of p21 and p27, protein folding after complex formation is required for SCFSkp2 to ligate ubiquitin40, 57,93. In other studies, the maximal affinity for p27 was recorded when the complete complex, including Cdk2 bound to Cks1, was formed87, 94. The structures of the CDKs can be taken from the crystal structures of the Cks1–Cdk2 complex (REF. 95), and the Cdk2–cyclin-A complex bound to a fragment of p27 that is amino-terminal to Thr187 (REF. 96), and can be superimposed to create a model of the Cks1–Cdk2–p27 complex (FIG. 2b). This model shows that p27 and Cks1 bind on opposite faces of Cdk2 that are separated by a distance that is almost equal to the length of Skp2 — although approximately 100 residues of p27 that are not present in the structure could easily make up the distance. In addition, the superimposition of the Skp2 LRR onto the β-Trcp WD-40 domain in the complete model clearly places the concave surface of the LRR (where p27 is expected to bind) some distance away from the equivalent location of the WD-repeat-bound VOLUME 5 | SEPTEMBER 2004 | 7 4 7 ©2004 Nature Publishing Group REVIEWS phosphorylated peptide (compare FIG. 2a,b). A model that was indicated by the recent structure of the carbohydrate-binding Fbx2 domain proposes that the substrate is bound in an equivalent region of space97. Does Skp2 bind the Cdk–p27–Cks1 complex en bloc such that the appropriate residues of p27 are placed into the same position as the substrates in the WD-40 and Fbx2 structures? The anticipated crystal structure of the Skp2–Cks1–p27 complex should clarify these Box 4 | Drug design at protein interfaces a b Chk1 SH2 domain β-Trcp WD-40 domain Some drugs that are already available target protein–protein interfaces119. Other studies have established that protein interfaces can be targeted by high-throughput screening with small molecules120–124 — notably, an Mdm2–p53 interaction inhibitor has been identified125. In addition, there are many examples of point mutations that disrupt protein interactions. Clearly, a small molecule that disrupts the position of one or two key residues might be equally effective. Nevertheless, there has historically been skepticism regarding targeting protein interfaces for drug discovery126,127. Much of this skepticism arose from the failure to find compounds that inhibit SH2-domain interactions despite intense efforts.Although point mutations were identified that disrupted the interaction surfaces of SH2 domains128, the search for drugs that could do the same failed. The three-dimensional structure of the targeted SH2 surface shows why: it is a flat protein surface with no pocket for a small molecule to bind (see figure part a, top panel). The secret might be that a combination of two factors is required for a small molecule to disrupt a protein interface: the compound must interact with a key residue and that key residue must be near or inside a protein cavity of suitable size and character to bind a drug-like compound. This combination of requirements might divide protein interfaces into those that are not, and more importantly, those that are susceptible to targeted drug discovery. The three-dimensional backbone structures of the SH2 domain from Chk1 (checkpoint kinase-1; see figure part a, top panel) and the β-Trcp WD-40 domain (see figure part b, top panel) show that these two interfaces are indeed shaped quite differently, with the latter surface appearing to be more suitable for drug discovery. In each structure, the largest drug-binding pocket at the protein-binding interface was found using the software program ICM PocketFinder (Molsoft Limited Liability Corporation, La Jolla, California, USA) and is displayed as a grey geometric object. The volumes of these pockets were calculated and plotted on a graph of volume (X axis) versus area (Y-axis; see the lower panels). The central region of the graph that corresponds to the volume/area distribution of most known drugs is coloured purple. The volume and area of the largest pocket on the surface of the SH2 domain (red square) falls outside of this region, whereas that formed by the central channel of the WD-40 domain in β-Trcp (red square) falls well within it. 748 | SEPTEMBER 2004 | VOLUME 5 interactions. But, the existing structures indicate an explanation for why free p21 and p27 (and Sic1) would not be efficiently ubiquitylated in the absence of complex formation with a CDK, as only the complex places its ubiquitin-attachment target location optimally into the hot zone. Interestingly, there is no reason why a high-affinity FBP–substrate interaction, in conjunction with the productive location of a non-Lys ubiquitinacceptor atom, might not result in non-Lys ubiquitylation in this model. Indeed, amino-terminal ubiquitylation of p21 has been shown98. So, the current data indicate that Skp2 uses a complicated combination of its surfaces and intramolecular interactions to accomplish the targeting of tricky, intrinsically disordered, posttranslationally modified proteins, even while they are intimately entwined with their constituent molecular machines. The design of related SCF ligases might therefore be suited to operation in molecular-machinery-rich environments such as the CDK-driven cell-cycle. The structural information on the SCF ligase illustrates the details of only one possible model of SCF function. Certain findings, however, hint at complementary or alternative models. For example, numerous FBPs have amino-terminal domains of unknown function, which were not present in any crystal structure; but in β-Trcp, for example, this domain is a homodimerization determinant99. These findings raise the possibility that oligomerization of the SCF complex is required for the substrate and ubiquitin to come together for ligation and ubiquitin-chain elongation to occur. Furthermore, kinetic studies indicate that E2–E3 interactions and E2 oligomerization might be significant determinants of polyubiquitin-chain formation100,101. The E2-binding site is not precisely known and seems to be strongly influenced by the addition of a Nedd8 moiety to the cullin near the E2-binding site102. The ubiquitin-like Nedd8 is removed for regulatory purposes by the COP9 61 SIGNALOSOME . Some SCF-active co-factors (for example, Cand1) bind to both the amino terminus (where Nedd8 is attached) and the carboxyl terminus of the cullin protein to regulate its activity (reviewed in REF. 61). This last observation, in particular, indicates the existence of intermediate forms of the SCF complex that are associated with cycles of Nedd8 ligation and removal in conjunction with Cand1–Cul1 binding cycles. Although such intermediates would be part of a model of SCF function that is alternative to the hot zone model, Cand1 might also turn out to provide a functional link between the E2-binding end of the SCF and the FBP end, without disturbing the rigid orientation that is seen in the crystal structures. Indeed, Nedd8 modification of the SCF dissociates Cand1 from Cul1, thereby allowing the Skp1–FBP complex to bind Cul1. Finally, the autoubiquitylation of FBPs that is observed in yeast103,104 and mammals105,106 would also favour the hot zone model, as the FBP is itself in the hot zone. Applied engineering of SCF ligases Evidence for the pathogenic involvement of SCF ligases in cancer and other proliferative processes continues to accumulate107. If these enzymes are truly a class of www.nature.com/reviews/molcellbio ©2004 Nature Publishing Group REVIEWS COP9 SIGNALOSOME An eight-subunit protein complex that regulates protein ubiquitylation and turnover in a variety of developmental and physiological contexts. Extensively characterized in plants but fundamental to all eukaryotes, this complex posttranslationally modifies the cullin subunit of E3-ubiquitin ligases by cleaving off the covalently coupled peptide, Nedd8. several E3 ligases that function as the checkpoint enablers of the cell cycle, this rich compendium of convergent structural and biological data might serve as an important asset for targeted drug discovery. An endogenous small-molecule-binding active site of the target is usually the focus of targeted drug discovery. The target site might be approximated by its activity in a scaleable assay in high-throughput screening, or it might be used in full atomic detail, as occurs in rational drug discovery. These active sites tend to be small, deep, rigid pockets in the three-dimensional structure of the protein. The SCF represents an unusual challenge in this regard: how can a small chemical compound competitively inhibit the large, protein-sized ‘active site’ of this unique enzyme? In theory, compounds that function as inhibitors could be designed to compete for binding the phosphorecognition sites of the FBP, as well as to allosteric pockets on this super-enzyme that affect its rigid angles of orientation. In both cases, the targeting of protein interfaces will be necessary — but, fortunately, some protein surfaces of the SCF have a valuable combination of features (BOX 4). The top of the narrow central channel of the doughnut-shaped WD-40 domain, for example, is also the site of the key residues that are involved in binding the phosphorylated peptide motif ( BOX 4, panel b). Several solvent cavities and pockets of unknown function can be found in some of the linker regions and at the E2 end of the cullin protein of the SCF structural models. The volume of these pockets is sufficient to accommodate small molecules that would be of an average size for known drugs. The targeted, structure-based discovery of small-molecule binders to some of these regions might 1. Guardavaccaro, D. & Pagano, M. Oncogenic aberrations of cullin-dependent ubiquitin ligases. Oncogene 23, 2037–2049 (2004). 2. Kraus, B. et al. A novel cyclin gene (CCNF) in the region of the polycystic kidney disease gene (PKD1). Genomics 24, 27–33 (1994). 3. Murray, A. W. Recycling the cell cycle: cyclins revisited. Cell 116, 221–234 (2004). 4. Bai, C., Richman, R. & Elledge, S. J. Human cyclin F. EMBO J. 13, 6087–6098 (1994). 5. Bai, C. et al. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell 86, 263–274 (1996). Seminal paper that identified and assigned a function to the F-box domain. 6. Feldman, R. M., Correll, C. C., Kaplan, K. B. & Deshaies, R. J. A complex of Cdc4p, Skp1p, and Cdc53p/cullin catalyzes ubiquitination of the phosphorylated CDK inhibitor Sic1p. Cell 91, 221–230 (1997). References 6 and 7 were the first studies to put the data together to identify the SCF ubiquitin ligase as a functional entity. 7. Skowyra, D., Craig, K. L., Tyers, M., Elledge, S. J. & Harper, J. W. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell 91, 209–219 (1997). 8. Cenciarelli, C. et al. Identification of a family of human F-box proteins. Curr. Biol. 9, 1177–1179 (1999). 9. Winston, J. T., Koepp, D. M., Zhu, C., Elledge, S. J. & Harper, J. W. A family of mammalian F-box proteins. Curr. Biol. 9, 1180–1182 (1999). 10. Smith, T. F., Gaitatzes, C., Saxena, K. & Neer, E. J. The WD repeat: a common architecture for diverse functions. Trends Biochem. Sci. 24, 181–185 (1999). 11. Kobe, B. & Kajava, A. V. The leucine-rich repeat as a protein recognition motif. Curr. Opin. Struct. Biol. 11, 725–732 (2001). result in either inhibitors (if they lock the complex into a rigid, inactive conformation) or activators (if they lock it into a rigid, active conformation). Most importantly, the lessons that are to be learnt from finding modulators of these super-enzymes — on the basis of these structural strategies for one FBP — should apply to many or all of the other super-enzymes that use the same cullin–Skp1–FBP version of the key interface, as well as to the development of protein interfaces for drug-discovery targeting in general. Conclusions The secrets of the SCF E3 ligase are being uncovered by a unique collection of data that spans human disease, cell biology, detailed structural biology and genomics. A clear picture of the SCF that operates with its upstream E1 and E2 enzymes and with the downstream 26S proteasome continues to emerge. The SCF itself might be seen as a fundamental post-translational effector in the cell — as shown by the diversity of phenotypes that are ascribed to FBPs and their broad evolutionary distribution. Examining the roles of SCF ligases through their effects on CDK activity reveals a surprisingly unified, but as yet incomplete, picture of the regulatory nuances in the mammalian cell cycle. The wealth of structural and biochemical data on this unique enzyme reveal why it is designed the way that it is and has already indicated drug-discovery approaches. These new investigations could begin to illuminate the role of the proteolytic compartment in cellular-proliferation-based human disease. The number and diversity of FBPs implies that this role will be expanded to a wide variety of developmental and organ-specific functions in humans. 12. Enkhbayar, P., Kamiya, M., Osaki, M., Matsumoto, T. & Matsushima, N. Structural principles of leucine-rich repeat (LRR) proteins. Proteins 54, 394–403 (2004). 13. Yoshida, Y. et al. E3 ubiquitin ligase that recognizes sugar chains. Nature 418, 438–442 (2002). 14. Yoshida, Y. et al. Fbs2 is a new member of the E3 ubiquitin ligase family that recognizes sugar chains. J. Biol. Chem. 278, 43877–43884 (2003). 15. Hsu, J. Y., Reimann, J. D., Sorensen, C. S., Lukas, J. & Jackson, P. K. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APCCdh1. Nature Cell Biol. 4, 358–366 (2002). 16. Reimann, J. D. et al. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell 105, 645–655 (2001). 17. Kawakami, K. et al. Proviral insertions in the zebrafish hagoromo gene, encoding an F-box/WD40-repeat protein, cause stripe pattern anomalies. Curr. Biol. 10, 463–466 (2000). 18. Sidow, A. et al. A novel member of the F-box/WD40 gene family, encoding dactylin, is disrupted in the mouse dactylaplasia mutant. Nature Genet 23, 104–107 (1999). 19. Basel, D., DePaepe, A., Kilpatrick, M. W. & Tsipouras, P. Split hand foot malformation is associated with a reduced level of Dactylin gene expression. Clin. Genet. 64, 350–354 (2003). 20. Ianakiev, P. et al. Split-hand/split-foot malformation is caused by mutations in the p63 gene on 3q27. Am. J. Hum. Genet. 67, 59–66 (2000). 21. Dias, D. C., Dolios, G., Wang, R. & Pan, Z. Q. CUL7: A DOC domain-containing cullin selectively binds Skp1–Fbx29 to form an SCF-like complex. Proc. Natl Acad. Sci. USA 99, 16601–16606 (2002). 22. Arai, T. et al. Targeted disruption of p185/Cul7 gene results in abnormal vascular morphogenesis. Proc. Natl Acad. Sci. USA 100, 9855–9860 (2003). NATURE REVIEWS | MOLECUL AR CELL BIOLOGY 23. Masuda, K. et al. Molecular profile of synovial fibroblasts in rheumatoid arthritis depends on the stage of proliferation. Arthritis Res. 4, R8 (2002). 24. den Engelsman, J., Keijsers, V., de Jong, W. W. & Boelens, W. C. The small heat-shock protein αB-crystallin promotes FBX4-dependent ubiquitination. J. Biol. Chem. 278, 4699–4704 (2003). 25. Sandri, M. et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117, 399–412 (2004). 26. Gomes, M. D., Lecker, S. H., Jagoe, R. T., Navon, A. & Goldberg, A. L. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl Acad. Sci. USA 98, 14440–14445 (2001). 27. Bodine, S. C. et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294, 1704–1708 (2001). 28. Musacchio, A. & Hardwick, K. G. The spindle checkpoint: structural insights into dynamic signalling. Nature Rev. Mol. Cell Biol. 3, 731–741 (2002). 29. Zachariae, W. & Nasmyth, K. Whose end is destruction: cell division and the anaphase-promoting complex. Genes Dev. 13, 2039–2058 (1999). 30. Peters, J. M. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell 9, 931–943 (2002). 31. Qu, Z., MacLellan, W. R. & Weiss, J. N. Dynamics of the cell cycle: checkpoints, sizers, and timers. Biophys. J. 85, 3600–3611 (2003). 32. Nakayama, K. et al. Degradation of p27 mediated by Skp2 is required for progression to mitosis. Dev. Cell 6, 661–672 (2004). Shows that p27 is one of the main substrates of SCFSkp2 — as the Skp2 –/– phenotype is rescued by p27 deficiency. It also shows that p27 inhibits Cdk1 as well as Cdk2. 33. Sherr, C. J. & Roberts, J. M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13, 1501–1512 (1999). VOLUME 5 | SEPTEMBER 2004 | 7 4 9 ©2004 Nature Publishing Group REVIEWS 34. Amati, B. & Vlach, J. Kip1 meets SKP2: new links in cellcycle control. Nature Cell Biol. 1, E91–E93 (1999). 35. Carrano, A. C., Eytan, E., Hershko, A. & Pagano, M. Skp2 is required for the ubiquitin-mediated degradation of the Cdkinhibitor p27. Nature Cell Biol. 1, 193–199 (1999). 36. Nakayama, K. et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27Kip1, polyploidy and centrosome overduplication. EMBO J. 19, 2069–2081 (2000). 37. Tsvetkov, L. M., Yeh, K. H., Lee, S. J., Sun, H. & Zhang, H. p27Kip1 ubiquitination and degradation is regulated by the SCFSkp2 complex through phosphorylated Thr187 in p27. Curr. Biol. 9, 661–664 (1999). 38. Sutterluty, H. et al. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nature Cell Biol. 1, 207–214 (1999). 39. Yu, Z. K., Gervais, J. & Zhang, H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21CIP1/WAF1 and cyclin D proteins. Proc. Natl Acad. Sci. USA 95, 11324–11329 (1998). 40. Bornstein, G. et al. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J. Biol. Chem. 278, 25752–25757 (2003). 41. Bashir, T., Dorrello, N. V., Amador, V., Guardavaccaro, D. & Pagano, M. Control of the SCFSkp2–Cks1 ubiquitin ligase by the APC/CCdh1 ubiquitin ligase. Nature 428, 190–193 (2004). References 41 and 42 established the biological ‘handshake’ between the main cell-cycle ubiquitin ligases. 42. Wei, W. et al. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature 428, 194–198 (2004). 43. Donzelli, M. et al. Dual mode of degradation of Cdc25 A phosphatase. EMBO J. 21, 4875–4884 (2002). 44. Wirth, K. G. et al. Loss of the anaphase-promoting complex in quiescent cells causes unscheduled hepatocyte proliferation. Genes Dev. 18, 88–98 (2004). Describes and confirms the phenotype of APC/C disruption. 45. Reimann, J. D., Gardner, B. E., Margottin-Goguet, F. & Jackson, P. K. Emi1 regulates the anaphase-promoting complex by a different mechanism than Mad2 proteins. Genes Dev. 15, 3278–3285 (2001). 46. Busino, L. et al. Degradation of Cdc25A by β-TrCP during S phase and in response to DNA damage. Nature 426, 87–91 (2003). 47. Jin, J. et al. SCFβ-TRCP links Chk1 signaling to degradation of the Cdc25A protein phosphatase. Genes Dev. 17, 3062–3074 (2003). 48. Koepp, D. M. et al. Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science 294, 173–177 (2001). 49. Moberg, K. H., Bell, D. W., Wahrer, D. C., Haber, D. A. & Hariharan, I. K. Archipelago regulates cyclin E levels in Drosophila and is mutated in human cancer cell lines. Nature 413, 311–316 (2001). 50. Strohmaier, H. et al. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature 413, 316–322 (2001). 51. Watanabe, N. et al. M-phase kinases induce phosphodependent ubiquitination of somatic Wee1 by SCFβ-TrCP. Proc. Natl Acad. Sci. USA 101, 4419–4424 (2004). 52. Ayad, N. G. et al. Tome-1, a trigger of mitotic entry, is degraded during G1 via the APC. Cell 113, 101–113 (2003). 53. Guardavaccaro, D. et al. Control of meiotic and mitotic progression by the F box protein β-Trcp1 in vivo. Dev. Cell 4, 799–812 (2003). 54. Margottin-Goguet, F. et al. Prophase destruction of Emi1 by the SCFβTrCP/Slimb ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Dev. Cell 4, 813–826 (2003). 55. Reed, S. I. Ratchets and clocks: the cell cycle, ubiquitylation and protein turnover. Nature Rev. Mol. Cell Biol. 4, 855–864 (2003). 56. Sheaff, R., Groudine, M., Gordon, M., Roberts, J. & Clurman, B. Cyclin E–Cdk2 is a regulator of p27Kip1. Genes Dev. 11, 1464–1478 (1997). 57. Montagnoli, A. et al. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 13, 1181–1189 (1999). 58. Vlach, J., Hennecke, S. & Amati, B. Phosphorylationdependent of the cyclin-dependent kinase inhibitor p27Kip1. EMBO J. 16, 5334–5344 (1997). 59. Welcker, M. et al. Multisite phosphorylation by Cdk2 and GSK3 controls cyclin E degradation. Mol. Cell 12, 381–392 (2003). 60. Moshe, Y., Boulaire, J., Pagano, M. & Hershko, A. Role of Polo-like kinase in the degradation of Emi1, a regulator of the anaphase promoting complex/cyclosome. Proc. Natl Acad. Sci. USA 101, 7937–7942 (2004). 750 61. Cope, G. A. & Deshaies, R. J. COP9 signalosome: a multifunctional regulator of SCF and other cullin-based ubiquitin ligases. Cell 114, 663–671 (2003). 62. Hon, W. C. et al. Structural basis for the recognition of hydroxyproline in HIF-1α by pVHL. Nature 417, 975–978 (2002). 63. Min, J. H. et al. Structure of an HIF-1α–pVHL complex: hydroxyproline recognition in signaling. Science 296, 1886–1889 (2002). 64. Schulman, B. et al. Insights into SCF ubiquitin ligases from the structure of the Skp1–Skp2 complex. Nature, 408, 381–386 (2000). The only crystallographic study so far on the substrate-binding portion of an FBL protein. 65. Stebbins, C. E., Kaelin, W. G. Jr. & Pavletich, N. P. Structure of the VHL–ElonginC–ElonginB complex: implications for VHL tumor suppressor function. Science 284, 455–461 (1999). 66. Wu, G. et al. Structure of a β-TrCP1–Skp1–β-catenin complex: destruction motif binding and lysine specificity of the SCFβ-TrCP1 ubiquitin ligase. Mol. Cell 11, 1445–1456 (2003). 67. Zheng, N. et al. Structure of the Cul1–Rbx1–Skp1–F boxSkp2 SCF ubiquitin ligase complex. Nature 416, 703–709 (2002). Reports the pivotal three-dimensional structural study of the SCF ligase. 68. Zheng, N., Wang, P., Jeffrey, P. D. & Pavletich, N. P. Structure of a c-Cbl–UbcH7 complex: RING domain function in ubiquitin-protein ligases. Cell 102, 533–539 (2000). 69. Huang, L. et al. Structure of an E6AP–UbcH7 complex: insights into ubiquitination by the E2–E3 enzyme cascade. Science 286, 1321–1326 (1999). 70. Verdecia, M. A. et al. Conformational flexibility underlies ubiquitin ligation mediated by the WWP1 HECT domain E3 ligase. Mol. Cell 11, 249–259 (2003). 71. Orlicky, S., Tang, X., Willems, A., Tyers, M. & Sicheri, F. Structural basis for phosphodependent substrate selection and orientation by the SCFCdc4 ubiquitin ligase. Cell 112, 243–256 (2003). 72. Joazeiro, C. A. et al. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science 286, 309–312 (1999). 73. Pintard, L. et al. The BTB protein MEL-26 is a substratespecific adaptor of the CUL-3 ubiquitin-ligase. Nature 425, 311–316 (2003). 74. Xu, L. et al. BTB proteins are substrate-specific adaptors in an SCF-like modular ubiquitin ligase containing CUL-3. Nature 425, 316–321 (2003). 75. Geyer, R., Wee, S., Anderson, S., Yates, J. & Wolf, D. A. BTB/POZ domain proteins are putative substrate adaptors for cullin 3 ubiquitin ligases. Mol. Cell 12, 783–790 (2003). 76. Furukawa, M., He, Y. J., Borchers, C. & Xiong, Y. Targeting of protein ubiquitination by BTB-Cullin 3-Roc1 ubiquitin ligases. Nature Cell Biol. 5, 1001–1007 (2003). 77. Hart, M. et al. The F-box protein β-TrCP associates with phosphorylated β-catenin and regulates its activity in the cell. Curr. Biol. 9, 207–210 (1999). 78. Kitagawa, M. et al. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of β-catenin. EMBO J. 18, 2401–2410 (1999). 79. Latres, E., Chiaur, D. S. & Pagano, M. The human F box protein β-Trcp associates with the Cul1/Skp1 complex and regulates the stability of β-catenin. Oncogene 18, 849–854 (1999). 80. Winston, J. T. et al. The SCFβ-TRCP ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 13, 270–283 (1999). 81. Petroski, M. D. & Deshaies, R. J. Context of multiubiquitin chain attachment influences the rate of Sic1 degradation. Mol. Cell 11, 1435–1444 (2003). Provides a detailed structure–function analysis of Sic1 degradation by an SCF. 82. Klein, P., Pawson, T. & Tyers, M. Mathematical modeling suggests cooperative interactions between a disordered polyvalent ligand and a single receptor site. Curr. Biol. 13, 1669–1678 (2003). 83. Lanker, S., Valdivieso, M. & Wittenberg, C. Rapid degradation of the G1 cyclin Cln2 induced by Cdkdependent phosphorylation. Science 271, 1597–1601 (1996). 84. Hsiung, Y. G. et al. F-box protein Grr1 interacts with phosphorylated targets via the cationic surface of its leucinerich repeat. Mol. Cell. Biol. 21, 2506–2520 (2001). 85. Spruck, C. et al. A CDK-independent function of mammalian Cks1: targeting of SCFSkp2 to the CDK inhibitor p27Kip1. Mol. Cell 7, 639–650 (2001). | SEPTEMBER 2004 | VOLUME 5 86. Ganoth, D. et al. The cell-cycle regulatory protein Cks1 is required for SCFSkp2-mediated ubiquitylation of p27. Nature Cell Biol. 3, 321–324 (2001). 87. Seeliger, M. A., Breward, S. E., Friedler, A., Schon, O. & Itzhaki, L. S. Cooperative organization in a macromolecular complex. Nature Struct. Biol. 10, 718–724 (2003). 88. Wang, W., Ungermannova, D., Chen, L. & Liu, X. A negatively charged amino acid in Skp2 is required for Skp2–Cks1 interaction and ubiquitination of p27Kip1. J. Biol. Chem. 278, 32390–32396 (2003). 89. Xu, K. et al. Protein–protein interactions involved in the recognition of p27 by E3 ubiquitin ligase. Biochem. J. 371, 957–964 (2003). 90. Bienkiewicz, E. A., Adkins, J. N. & Lumb, K. J. Functional consequences of preorganized helical structure in the intrinsically disordered cell-cycle inhibitor p27Kip1. Biochemistry 41, 752–759 (2002). 91. Kriwacki, R. W., Hengst, L., Tennant, L., Reed, S. I. & Wright, P. E. Structural studies of p21Waf1/Cip1/Sdi1 in the free and Cdk2-bound state: conformational disorder mediates binding diversity. Proc. Natl Acad. Sci. USA 93, 11504–11509 (1996). 92. Adkins, J. N. & Lumb, K. J. Intrinsic structural disorder and sequence features of the cell cycle inhibitor p57Kip2. Proteins 46, 1–7 (2002). 93. Lacy, E. R. et al. p27 binds cyclin–CDK complexes through a sequential mechanism involving binding-induced protein folding. Nature Struct. Mol. Biol. 11, 358–364 (2004). 94. Sitry, D. et al. Three different binding sites of Cks1 are required for p27-ubiquitin ligation. J. Biol. Chem. 277, 42233–42240 (2002). 95. Bourne, Y. et al. Crystal structure and mutational analysis of the human CDK2 kinase complex with cell cycle-regulatory protein CksHs1. Cell 84, 863–874 (1996). 96. Russo, A. A., Jeffrey, P. D., Patten, A. K., Massague, J. & Pavletich, N. P. Crystal structure of the p27Kip1 cyclindependent-kinase inhibitor bound to the cyclin A–Cdk2 complex. Nature 382, 325–331 (1996). Provides the most complete set of structural data on p27. 97. Mizushima, T. et al. Structural basis of sugar-recognizing ubiquitin ligase. Nature Struct. Mol. Biol. 11, 365–370 (2004). 98. Bloom, J., Amador, V., Bartolini, F., DeMartino, G. & Pagano, M. Proteasome-mediated degradation of p21 via N-terminal ubiquitylation. Cell 115, 71–82 (2003). 99. Suzuki, H. et al. IκBα ubiquitination is catalyzed by an SCF-like complex containing Skp1, cullin-1, and two F-box/WD40-repeat proteins, βTrCP1 and βTrCP2. Biochem. Biophys. Res. Commun. 256, 127–132 (1999). 100. Deffenbaugh, A. E. et al. Release of ubiquitin-charged Cdc34-SUb from the RING domain is essential for ubiquitination of the SCFCdc4-bound substrate Sic1. Cell 114, 611–622 (2003). 101. Varelas, X., Ptak, C. & Ellison, M. J. Cdc34 self-association is facilitated by ubiquitin thiolester formation and is required for its catalytic activity. Mol. Cell. Biol. 23, 5388–5400 (2003). 102. Pan, Z. Q., Kentsis, A., Dias, D. C., Yamoah, K. & Wu, K. Nedd8 on cullin: building an expressway to protein destruction. Oncogene 23, 1985–1997 (2004). 103. Kus, B. M., Caldon, C. E., Andorn-Broza, R. & Edwards, A. M. Functional interaction of 13 yeast SCF complexes with a set of yeast E2 enzymes in vitro. Proteins 54, 455–467 (2004). 104. Zhou, P. & Howley, P. M. Ubiquitination and degradation of the substrate recognition subunits of SCF ubiquitin-protein ligases. Mol. Cell 2, 571–580 (1998). 105. Wirbelauer, C. et al. The F-box protein Skp2 is a ubiquitylation target of a Cul1-based core ubiquitin ligase complex: evidence for a role of Cul1 in the suppression of Skp2 expression in quiescent fibroblasts. EMBO J. 19, 5362–5375 (2000). 106. Li, Y., Gazdoiu, S., Pan, Z. Q. & Fuchs, S. Y. Stability of homologue of slimb F-box protein is regulated by availability of its substrate. J. Biol. Chem. 279, 11074–11080 (2004). 107. Pagano, M. & Benmaamar, R. When protein destruction runs amok, malignancy is on the loose. Cancer Cell 4, 251–256 (2003). 108. Bashir, T. & Pagano, M. Aberrant ubiquitin-mediated proteolysis of cell cycle regulatory proteins and oncogenesis. Adv. Cancer Res. 88, 101–144 (2003). 109. Pickart, C. M. Back to the future with ubiquitin. Cell 116, 181–190 (2004). 110. Hershko, A., Ciechanover, A. & Varshavsky, A. Basic Medical Research Award. The ubiquitin system. Nature Med. 6, 1073–1081 (2000). www.nature.com/reviews/molcellbio ©2004 Nature Publishing Group REVIEWS 111. Pickart, C. M. & Cohen, R. E. Proteasomes and their kin: proteases in the machine age. Nature Rev. Mol. Cell Biol. 5, 177–187 (2004). 112. Liu, Y. C. Ubiquitin ligases and the immune response. Annu. Rev. Immunol. 22, 81–127 (2004). 113. Kipreos, E. T. & Pagano, M. The F-box protein family. Genome Biol. 1, REVIEWS3002 (2000). 114. Nayak, S. et al. The Caenorhabditis elegans Skp1-related gene family: diverse functions in cell proliferation, morphogenesis, and meiosis. Curr. Biol. 12, 277–287 (2002). 115. Yamanaka, A. et al. Multiple Skp1-related proteins in Caenorhabditis elegans: diverse patterns of interaction with Cullins and F-box proteins. Curr. Biol. 12, 267–275 (2002). 116. Harmon, F. G. & Kay, S. A. The F box protein AFR is a positive regulator of phytochrome A-mediated light signaling. Curr. Biol. 13, 2091–2096 (2003). 117. Marrocco, K., Lecureuil, A., Nicolas, P. & Guerche, P. The Arabidopsis SKP1-like genes present a spectrum of expression profiles. Plant Mol. Biol. 52, 715–727 (2003). 118. Risseeuw, E. P. et al. Protein interaction analysis of SCF ubiquitin E3 ligase subunits from Arabidopsis. Plant J. 34, 753–767 (2003). 119. Owellen, R. J., Hartke, C. A., Dickerson, R. M. & Hains, F. O. Inhibition of tubulin-microtubule polymerization by drugs of the Vinca alkaloid class. Cancer Res. 36, 1499–1502 (1976). 120. Baba, M. et al. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl Acad. Sci. USA 96, 5698–5703 (1999). 121. Arkin, M. R. et al. Binding of small molecules to an adaptive protein–protein interface. Proc. Natl Acad. Sci. USA 100, 1603–1608 (2003). 122. Berg, T. et al. Small-molecule antagonists of Myc/Max dimerization inhibit Myc-induced transformation of chicken embryo fibroblasts. Proc. Natl Acad. Sci. USA 99, 3830–3835 (2002). 123. Chen, J. K., Taipale, J., Young, K. E., Maiti, T. & Beachy, P. A. Small molecule modulation of Smoothened activity. Proc. Natl Acad. Sci. USA 99, 14071–14076 (2002). 124. Lepourcelet, M. et al. Small-molecule antagonists of the oncogenic Tcf/β-catenin protein complex. Cancer Cell 5, 91–102 (2004). 125. Vassilev, L. T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004). This break-through drug-discovery work established that protein interfaces can be disrupted by small molecules, and that ubiquitin ligases can be targeted. 126. Toogood, P. L. Inhibition of protein–protein association by small molecules: approaches and progress. J. Med. Chem. 45, 1543–1558 (2002). 127. Cochran, A. G. Protein–protein interfaces: mimics and inhibitors. Curr. Opin. Chem. Biol. 5, 654–659 (2001). 128. Mayer, B. J. & Baltimore, D. Mutagenic analysis of the roles of SH2 and SH3 domains in regulation of the Abl tyrosine kinase. Mol. Cell. Biol. 14, 2883–2894 (1994). Acknowledgments We thank T. Hunter and N. Watanabe for communicating results prior to publication. We apologize to colleagues whose work could not be mentioned due to space limitations. M.P. is grateful to T. M. Thor for continuous support. Molecular graphics were produced by ICM software (Molsoft Limited Liability Corporation, La Jolla, California, USA). Work in the Pagano laboratory is supported by grants from the National Institutes of Health. NATURE REVIEWS | MOLECUL AR CELL BIOLOGY Competing interests statement The authors declare that they have no competing financial interests. Online links DATABASES The following terms in this article are linked online to: Entrez: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi CCNF InterPro: http://www.ebi.ac.uk/interpro/ F-box domain | HECT domain | LRR | RING-finger domain OMIM: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM split hand–foot malformation Saccharomyces genome database: http://www.yeastgenome.org/ Cdc4 | Cdc20 | Sic1 Swiss-Prot: http://us.expasy.org/sprot/ Cul1 | Fbw1 | Fbw4 | Fbw7 | Fbx2 | Fbx3 | Fbx4 | Fbx5 | Fbx6 | Fbx32 | Nedd8 | p21 | p27 | Rbx1 | Skp1 | Skp2 | Ubc3 | VHL FURTHER INFORMATION HGNC F-box gene family nomenclature http://www.gene.ucl.ac.uk/nomenclature/genefamily/ FBX.shtml Pfam database: http://pfam.wustl.edu/cgi-bin/getdesc?name=F-box InterPro database: http://www.ebi.ac.uk/interpro/ Michele Pagano’s laboratory: http://www.med.nyu.edu/Path/Pagano/ Access to this links box is available online. VOLUME 5 | SEPTEMBER 2004 | 7 5 1 ©2004 Nature Publishing Group