* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Glycogen Metabolism Gluconeogenesis
Metalloprotein wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Biochemical cascade wikipedia , lookup
Biosynthesis wikipedia , lookup
Mitogen-activated protein kinase wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Western blot wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Proteolysis wikipedia , lookup
Paracrine signalling wikipedia , lookup
Blood sugar level wikipedia , lookup
Lipid signaling wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Signal transduction wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Citric acid cycle wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
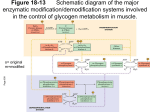
Glycogen Metabolism and Gluconeogenesis CH 339K Glycolysis (recap) •We discussed the reactions which convert glucose to pyruvate: C6H12O6 +2 NAD+ + 2 ADP → 2 CH3COCOOH + 2 NADH +2 ATP + 2 H+ •What about the sources of glucose? – Dietary sugars – Glycogen Before we get to glycogen: Dietary sugars Glycogen • Branched every 8-12 residues • Up to 50,000 or so residues total Breakdown: Glycogen Phosphorylase Glycogen Synthesis and Breakdown • Glycogen synthesis and breakdown are both controlled by hormones • Glucagon, Epinephrine – turn on glycogen breakdown – Turn off glycogen synthesis • Hormones act through receptors on cell surface and G-proteins Glucagon – 29 amino acid polypeptide produced in pancreas in response to low blood sugar Epinephrine – aka adrenaline – produced by adrenal medulla in response to stress Activation of Glycogen Phosphorylase 3’-5’ cyclic AMP G-Proteins G proteins are heterotrimers, containing Gα, Gβ and Gγ subunits. Subunit Size Ga 45 – 47 kD Gβ 35 kD Gg 7-9 kD G-Proteins • The Gα subunits bind guanine nucleotides (hence the name “G Protein”). G Proteins are associated on one hand with the inner surface of the plasma membrane, and on the other hand with membrane spanning receptor proteins called G-protein coupled receptors or GPCRs. • There are a number of different GPCRs; most commonly these are receptors for hormones or for some type of extracellular signal. • In the “resting” state, Gα is bound to the Gβ-Gγ dimer. Gα contains the nucleotide binding site, holding GDP in the inactive form, and is the “warhead” of the G protein. At least 20 different forms of Ga exist in mammalian cells. • Binding of the extracellular signal by the GPCR causes it to undergo an intracellular conformational change; this causes an allosteric effect on Gα. The change in Gα causes it to exchange GDP for GTP. GTP activates Gα, causing it to dissociate from the Gβ-Gγ dimer. The activated Gα binds and activates an effector molecule. • Gα also has a slow GTPase activity. Hydrolysis of GTP deactivates Gα, which reassociates with the Gβ-Gγ dimer and the GPCR to reform the resting state. In other words, G-protein mediated cellular responses have a built-in off switch to prevent them from running forever. G-Protein Coupled Receptors (GPCRs) G-Proteins – Effect of GDP/GTP Binding GDP – missing terminal PO4 allowss the βγbinding loop (red) to assime a looser conformation GTP – terminal PO4 constrains the βγ-binding loop (red) Cycling of G protein between active and inactive states G-Protein Killers Cholera Cholera toxin secreted by the bacterium Vibrio cholera. A subunit and five B subunits. A subunit catalyzes the transfer of an ADP-ribose from NAD+ to a specific Arg side chain of the α subunit of Gs. Gα is irreversibly modified by addition of ADP-ribosyl group; Modified Gα can bind GTP but cannot hydrolyze it ). As a result, there is an excessive, nonregulated rise in the intracellular cAMP level (100 fold or more), which causes a large efflux of Na+ and water into the gut. Pertussis (whooping cough) Pertussis toxin (secreted by Bordetella pertussis) catalyzes ADP-ribosylation of a specific cysteine side chain on the α subunit of a G protein which inhibits adenyl cyclase and activates sodium channels. This covalent modification prevents the subunit from interacting with receptors; as a result, locking Gα in the GDP bound form. You probably vaccinate your dog against the related species that causes kennel cough. Cholera is still a problem2009 Zimbabwe outbreak – 4300 deaths Activation of Adnylate Cyclase Activation of cAMP-Dependant Protein Kinase Glycogen Phosphorylase • Exists in 2 forms – Phosphorylase B (inactive) – Phosphorylase A (active) • Phosphorylase B is converted to Phosphorylase A when it is itself phosphorylated by Synthase Phosphorylase Kinase (SPK) • GP cannot remove branch points (α-1,6 linkages) Activation of Glycogen Phosphorylase cAMP – dependent Protein Kinase 3’-5’ cyclic AMP Activation of Glycogen Phosphorylase cAMP – dependent Protein Kinase Debranching Enzyme • The activity of phosphorylase ceases 4 glucose residues from the branch point. • Debranching enzyme (also called glucan transferase) contains 2 activities: – glucotransferase – glucosidase. • Glycogenolysis occurring in skeletal muscle could generate free glucose which could enter the blood stream. • However, the activity of hexokinase in muscle is so high that any free glucose is immediately phosphorylated and enters the glycolytic pathway. Cori Disease • Cori disease (Glycogen storage disease Type III) is characterized by accumulation of glycogen with very short outer branches, caused by a flaw in debranching enzyme. • Deficiency in glycogen debranching activity causes hepatomegaly, ketotic hypoglycemia, hyperlipidemia, variable skeletal myopathy, cardiomyopathy and results in short stature. Glycogen Synthesis • Glycogen Synthase adds glucose residues to glycogen • Synthase cannot start from scratch – needs a primer • Glycogenin starts a new glycogen chain, bound to itself Glycogen Synthesis (cont.) • Synthase then adds to the nonreducing end. Glycogen Synthesis (cont.) • To add to the glycogen chain, synthase uses an activated glucose, UDPGlucose • UDP-Glucose Pyrophosphorylase links UDP to glucose Glycogen Synthesis (cont.) • Synthase then adds the activated glucose to the growing chain • Release and subsequent hydrolysis of pyrophosphate drives the reaction to the right Glycogen Synthesis (cont.) • Glycogen branching enzyme then introduces branch points Mature Glycogen • Built around glycogenin core • Multiple nonreducing ends accessible to glycogen phosphorylase Reverse Regulation of Phosphorylase and Synthase • The same kinase phosphorylates both phosphorylase and synthase • Synthase A (dephos.) is always active • Synthase D (phos.) is dependent on [G-6-P] • The same event that turns one on turns the other one off. Gluconeogenesis CH 339K Gluconeogenesis • Average adult human uses 120 g/day of glucose, mostly in the brain (75%) – About 20g glucose in body fluids – About 190 g stored as glycogen – Less than 2 days worth • • • • In addition to eating glucose, we also make it Mainly occurs in liver (90%) and kidneys (10%) Not the reverse of glycolysis Differs at the irreversible steps in glycolysis Gluconeogenesis Differs Here And Here And Here First Difference Glycolysis: make a nucleotide triphosphate Gluconeogenesis: burn two nucleotide triphosphates Pyruvate Carboxylase PEP Carboxykinase Malate Shuttle • Pyruvate Carboxylase is mitochondrial • OAA reduced to malate in matrix • Carrier transports malate to cytoplasm • Cytoplasmic malate dehydrogenase reoxidizes to OAA Second and Third differences Energetics Gluconeogenesis • Pyruvate + 4 ATP + 2 GTP + 2 NADH + 2 H2O ⇌ glucose + 4 ADP + 2 GDP + 2 NAD+ •ΔG = -37 kJ/mol Glycolysis (reversed) • Pyruvate + 2 ATP + 2 NADH + 2 H2O ⇌ glucose + 2 ADP + 2 NAD+ •ΔG = +84 kJ/mol Net difference of 4 nucleotide triphosphate bonds at ~31 kJ each accounts for difference in ΔGs Local Regulation • Phosphofructokinase-1(Glycolysis) is inhibited by ATP and Citrate and stimulated by AMP. • Fructose-1,6-bisphosphatase (Gluconeogenesis) is inhibited by AMP. Global Control Enzymes relevant to these pathways that are phosphorylated by cAMP-Dependent Protein Kinase include: • Pyruvate Kinase, a glycolysis enzyme that is inhibited when phosphorylated. • A bi-functional enzyme that makes and degrades an allosteric regulator, fructose-2,6bisphosphate. Pyruvate Kinase Regulation • Local regulation by substrate activation • Global regulation by hormonal control of Protein Kinase A Effects of Fructose-2,6-Bisphosphate • Fructose-2,6-bisphosphate allosterically activates the glycolysis enzyme Phosphofructokinase-1, promoting the relaxed state, even at relatively high [ATP]. Activity in the presence of fructose-2,6bisphosphate is similar to that observed when [ATP] is low. Thus control by fructose-2,6-bisphosphate, whose concentration fluctuates in response to external hormonal signals, supercedes control by local conditions (ATP concentration). • Fructose-2,6-bisphosphate instead inhibits the gluconeogenesis enzyme Fructose-1,6-bisphosphatase. Source of Fructose-2,6-Bisphosphate Fructose-2,6-bisphosphate is synthesized and degraded by a bi-functional enzyme that includes two catalytic domains • Phosphofructokinase-2 (PFK2) domain catalyzes: fructose-6-phosphate + ATP ⇄ fructose-2,6-bisphosphate + ADP. • Fructose-Biosphosphatase-2 (FBPase2) domain catalyzes: fructose-2,6-bisphosphate + H2O ⇄ fructose-6-phosphate + Pi. Phosphorylation activates FBPase2 and inhibits PFK2 BifunctionalEnzyme Activates PFK1 Inhibits F-1,6-bisphosphatase Inhibits PFK1 Activates F-1,6-bisphosphatase Medical aside – nonlethal! People with Type II diabetes have very high (~3x normal) rates of gluconeogenesis Initial treatment is usually with metformin. Metformin shuts down production of PEPCK and Glucose-6-phosphatase, inhibiting gluconeogenesis. Futile Cycles • Occur when loss of reciprocal regulation fails twixt glycolysis and gluconeogenesis • Anesthestics like halothane occasionally lead to runaway cycle between PFK and fructose-1,6-BPase • Malignant Hyperthermia The Cori Cycle High NADH/NAD+ Low NADH/NAD+