* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Powerpoint file - revised
RNA silencing wikipedia , lookup
RNA interference wikipedia , lookup
Community fingerprinting wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Bottromycin wikipedia , lookup
Molecular evolution wikipedia , lookup
Polyadenylation wikipedia , lookup
Biochemistry wikipedia , lookup
Secreted frizzled-related protein 1 wikipedia , lookup
Histone acetylation and deacetylation wikipedia , lookup
Gene expression profiling wikipedia , lookup
List of types of proteins wikipedia , lookup
Non-coding DNA wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Messenger RNA wikipedia , lookup
Transcription factor wikipedia , lookup
Point mutation wikipedia , lookup
Non-coding RNA wikipedia , lookup
Expression vector wikipedia , lookup
Epitranscriptome wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Gene regulatory network wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Gene expression wikipedia , lookup
Eukaryotic transcription wikipedia , lookup
RNA polymerase II holoenzyme wikipedia , lookup
Promoter (genetics) wikipedia , lookup
Transcriptional regulation wikipedia , lookup
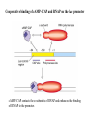
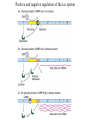
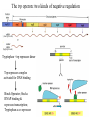
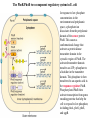
Regulation of prokaryotic transcription 1. Single-celled organisms with short doubling times must respond extremely rapidly to their environment. 2. Half-life of most mRNAs is short (on the order of a few minutes). 3. Coupled transcription and translation occur in a single cellular compartment. Therefore, transcriptional initiation is usually the major control point. Most prokaryotic genes are regulated in units called operons (Jacob and Monod, 1960) Operon: a coordinated unit of gene expression consisting of one or more related genes and the operator and promoter sequences that regulate their transcription. The mRNAs thus produced are “polycistronic’—multiple genes on a single transcript. The metabolism of lactose in E. coli & the lactose operon •To use lactose as an energy source, cells must contain the enzyme b-galactosidase. •Utilization of lactose also requires the enzyme lactose permease to transport lactose into the cell. •Expression of these enzymes is rapidly induced ~1000-fold when cells are grown in lactose compared to glucose. Transglycosylation LacZ: b-galactosidase; Y: galactoside permease; A: transacetylase (function unknown). P: promoter; O: operator. LacI: repressor; PI and LacI are not part of the operon. QuickTime™ and a GIF decompressor are needed to see this picture. IPTG: nonmetabolizable artificial inducer (can’t be cleaved) Negative regulation of the lac operon Negative regulation: The product of the I gene, the repressor, blocks the expression of the Z, Y, and A genes by interacting with the operator (O). The inducer (lactose or IPTG) can bind to the repressor, which induces a conformational change in the repressor, thereby preventing its interaction with the operator (O). When this happens, RNA polymerase is free to bind to the promoter (P) and initiates transcription of the lac genes. Symmetry matching between the tetramers of lac repressor and the nearly palindromic sequence of the lac operator Each monomeric unit of lacI is 37-kD The lac operator sequence is a nearly perfect inverted repeat centered around the GC base pair at position + 11. Regulation of the lac operon involves more than a simple on/off switch provided by lacI/lacO Observation: Glucose is a preferred sugar for E. coli, which uses glucose and ignores lactose in media containing both sugars. In these cells, b-galactosidase level is low, suggesting that derepression at the operator site is not enough to turn on the lac operon. This phenomenon is called catabolite repression. Catabolite control of the lac operon (a) Under conditions of high glucose, a glucose breakdown product inhibits the enzyme adenylate cyclase, preventing the conversion of ATP into cAMP. (b) As E. coli becomes starved for glucose, there is no breakdown product, and therefore adenylate cyclase is active and cAMP is formed. (c) When cAMP (a hunger signal) is present, it acts as an allosteric effector, complexing with the CAP dimer. CAP sites are also present in other promoters. cAMP-CAP is a global catabolite gene activator. (d) The cAMP-CAP complex (not CAP alone) acts as an activator of lac operon transcription by binding to a region within the lac promoter. (CAP = catabolite activator protein; cAMP = cyclic adenosine monophosphate) X-ray structure of CAPcAMP bound to DNA Cooperative binding of cAMP-CAP and RNAP on the lac promoter cAMP-CAP contacts the a-subunits of RNAP and enhances the binding of RNAP to the promoter. Positive and negative regulation of the lac operon More surprises about the regulation of the lac operon •The tetrameric lac repressor binds to the primary lac operator (O1) and one of two secondary operators (O2 or O3) simultaneously. The two structures are in equilibrium. The secondary operators function to increase the local concentration of lac repressor (~ 10 per cell) in the micro-vicinity of the primary operator. When both O2 and O3 are mutated, repression at the lac promoter is reduced by ~70-fold. Mutation of only O2 or O3 reduces repression 2-fold. The trp operon: two kinds of negative regulation (low trp levels) (high trp levels) Tryptophan + trp repressor dimer Trp-repressor complex activated for DNA binding Binds Operator; blocks RNAP binding & represses transcription; Tryptophan a co-repressor Translation of part of the leader mRNA to produce the leader peptide What is theisattenuator? The attenuator a Rho-independent transcription terminator! Presence of Trp codons within the leader peptide is highly significant! Attenuation is mediated by the tight coupling of transcription and translation •The ribosome translating the trp leader mRNA follows closely behind the RNA polymerase that is transcribing the DNA template. •Alternative conformation adopted by the leader mRNA. completed UUU 3’ and blocking sequence 2 Incomplete leader peptide •The stalled ribosome is waiting for tryptophanyltRNA. •The 2:3 pair is not an attenuator and is more stable than the 3:4 pair. Two-step decoding process for translating nucleic acid sequences in mRNA into amino acid sequences in proteins The PhoR/PhoB two-component regulatory system in E. coli In response to low phosphate concentrations in the environment and periplasmic space, a phosphate ion dissociates from the periplasmic domain of the sensor protein PhoR. This causes a conformational change that activates a protein kinase transmitter domain in the cytosolic region of PhoR. The activated transmitter domain transfers an ATP -phosphate to a histidine in the transmitter domain. This phosphate is then transferred to an aspartic acid in the response regulator PhoB. Phosphorylated PhoB then activate transcription from genes encoding proteins that help the cell to respond to low phosphate, including phoA, phoS, phoE, and ugpB. Activation of 54-containing RNA polymerase at glnA promoter by NtrC (kinase) •The glnA gene encodes glutamine synthetase, which synthesizes glutamine from glutamic acid and ammonia. • The 54-containing RNA polymerase binds to the glnA promoter, forming a closed complex, before being activated. • In response to low levels of glutamine, a protein kinase called NtrB phosphorylates dimeric NtrC, which then binds to two sequence elements (called enhancer) located at –108 and -140. • The bound phosphorylated NtrC dimers interact with the bound 54-polymerase, causing the intervening DNA to form a loop. • The ATPase activity of NtrC then stimulates the polymerase to unwind the template strands at the start site, forming an open complex. Transcription of the glnA gene can then begin. DNA looping permits interaction between bound 54-RNA polymerase and NtrC