* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download week 3 ppt
Neurophilosophy wikipedia , lookup
Neuroplasticity wikipedia , lookup
Neuroeconomics wikipedia , lookup
History of neuroimaging wikipedia , lookup
Brain Rules wikipedia , lookup
Environmental enrichment wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Neuropsychology wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Signal transduction wikipedia , lookup
Impact of health on intelligence wikipedia , lookup
Neuroanatomy wikipedia , lookup
Metastability in the brain wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Haemodynamic response wikipedia , lookup
Aging brain wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Alzheimer's disease wikipedia , lookup
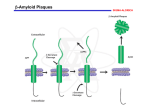
Alzheimer’s Disease (AD) Alzheimer’s Disease? • Alzheimer's disease (AD), also known as Senile Dementia of the Alzheimer Type (SDAT) or simply Alzheimer’s is the most common form of dementia. This incurable, degenerative, terminal disease was first described by a German psychiatrist and neuropathologist Alois Alzheimer in 1906 and was named after him. • Alzheimer's disease (AD) is a slowly progressive neurodegenerative disorder of the brain mostly affects the elderly and characterized by impairment of memory and eventually by disturbances in reasoning, planning, language, and perception. • Many scientists believe that Alzheimer's disease results from an increase in the production or accumulation of a specific protein (beta-amyloid protein) in the brain that leads to nerve cell death. • Generally, it is diagnosed in people over 65 years of age. AD Signs & Symptoms: • Memory loss for recent events or new information. • Progresses into dementia almost total memory loss • Inability to converse, loss of language ability Confirmation of Diagnosis: • Neuronal (amyloid, b amyloid protein, Ab amyloid) plaques • Neurofibrillary tangles • Brain Atrophy, Brain shrinkage (loss of neurons mainly in the hippocampus and basal forebrain) Stages of AD Brain Atrophy in AD WRONG! http://abdellab.sunderland.ac.uk/lectures/Neurodegeneration/References/Brain_Neurons_AD_Normal.html The Anatomical Hallmark of Alzheimer’s Pathology: Amyloid Plaques and Neurofibrillary Tangles in Brain Amyloid Plaques contain large amounts of a 42 amino acid peptide termed “b-amyloid”, or Ab42 b-amyloid itself is the initial cause of the pathophysiology that leads to dementia. Neurofibrillary tangles: rich in cytoskeletal proteins, especially the microtubule-associated protein, “tau”. In the tangles: heavily phosphorylated proteins, which may cause aggregation and precipitation of the cytoskeleton. Also generally reduced brain volume, especially in entorhinal cortex and hippocampus 9 From http://www.rnw.nl/health/html/brain.html Structure of amyloid precursor protein (APP) b-secretase Pathway: (not drawn to scale) APP Protein: b a g g (1) b-secretase cuts APP protein, giving: (2) g-secretase cuts this residue, giving: Ab40 Fragment Soluble Ab42 Fragment Unsoluble, aggregates into plaques or Amyloid precursor protein (APP) is membrane protein that sits in the membrane and extends outward. It is though to be important for neuronal growth, survival, and repair. From: www.niapublications.org/pubs/unraveling/01.htm Enzymes cut the APP into fragments, the most important of which for AD is called b-amyloid (beta-amyloid) or Ab. From: www.niapublications.org/pubs/unraveling/01.htm Beta-amyloid is “sticky” so the fragments cling together along with other material outside of the cell, forming the plaques seen in the AD brain. From: www.niapublications.org/pubs/unraveling/01.htm Processing of APP Two Major Hypotheses for AD: b amyloid protein (BAP) v. tau 1. BAPtists: The accumulation of a fragment of the amyloid precursor protein or APP (the amyloid beta 42 residue fragment or Ab-42) leads to the formation of plaques that kill neurons. 2. TAUists: Abnormal phosphorylation of tau proteins makes them “sticky,” leading to the break up of microtubules. The resulting loss of axonal transport causes cell death. Amyloid Hypothesis (it’s the plaques) 1. The amyloid precursor protein (APP) is broken down by a series of secretases (see previous two slides). 2. During this process, a nonsoluble fragment of the APP protein (called Ab42) accumulates and is deposited outside the cell. 3. The nonsoluble or “sticky” nature of Ab-42 helps other protein fragments (including apoE) to gather into plaques. 4. Somehow the plaques (or possible the migration of Ab-42 outside the cell) cause neuronal death. 5. PSEN1 & PSEN2 genes subunits of g secretase. Theories of How Damage Occurs in AD From Inside the Cell: Tangle Formation Axon Microtubules Tangles Neuron Tau Proteins Paired Helical Filament Dendrites Tau proteins, which normally stabilize microtubules in brain cells... undergo abnormal chemical changes and assemble into spirals called paired helical filaments... thus creating tangles that disrupt cell functions and lead to cell death. Sources: Dr John Trojanowski and Dr Virginia M. Y. Lee. University of Pennsylvania Medical Center. 18 Microtubules are like railroad tracks that transport nutrition and other molecules. Tau-proteins act as “ties” that stabilize the structure of the microtubules. In AD, tau proteins become tangled, unstabilizing the structure of the microtubule. Loss of axonal transport results in cell death. • Loss of cholinergic neurons in the basal forebrain nuclei; therefore, restoring cholinergic function might be helpful. • Choline acetyltransferase (CAT) activity is reduced in cortex and hippocampus. • Nicotinic, but not muscarinic, receptors are reduced. Therapeutic approaches 1. Cholinesterase inhibitors: The first drugs approved for treating AD. Enhancement of cholinergic transmission might compensate for the cholinergic deficit. Improve cognitive performance. 2. Inhibiting excitotoxicity 3. Inhibiting neurodegeneration (future target). Cholinesterase inhibitors 1. Tacrine • • • • The first drug approved for treating AD. Four times daily Cholinergic side effects (nausea and abdominal cramps) Hepatotoxicity Cholinesterase inhibitors 2. Donepezil (no hepatotoxicity) 3. Rivastigmine: Long-lasting drug, CNS selective (fewer peripheral cholinergic side effects). 4. Galantamine: Acts partly by cholinesterase inhibitor and partly by activation of brain nicotinic AChRs. Inhibiting excitotoxicity • Memantine – a use-dependent blockade of NMDA receptors. Additional Treatments for AD Role of dietary factors • Low saturated fat diets, • Vitamin E – decrease cytotoxicity - may slow the progression of the disease Cholinergic stimulation: • Nicotine patch, varenicline (Chantix) Pharmacologic Options for AD • Cognitive enhancers; can improve cognition and functional ability ─ 2 classes • Cholinesterase inhibitors (ChEIs) • NMDA-receptor antagonist Treatment of AD: Cognitive Enhancers Drug Name Dosage form Indication Donepezil (Aricept) Tablet, orally disintegrating table Mild to severe Galantamine (Razadyne®) Tablet/oral solution Mild to moderate Rivastigmine (Exelon®) Capsule Memantine (Namenda®) Tablet*, oral solution* Extended-release capsule* Mild to moderate Patch Mod to severe Common Side Effects NMDA-receptor antagonist Cholinesterase inhibitors (ChEIs) (Donepezil, galantamine, rivastigmine) • • • • • • Nausea Vomiting Diarrhea Weight loss Loss of appetite Muscle weakness (Memantine) • • • • Dizziness Headache Constipation Confusion National Institute on Aging. Alzheimer’s disease medications. November 2008. NIH Publication No. 08-3431. Available at: http://www.nia.nih.gov/Alzheimers/Publications/medicationsfs.htm. Accessed July 24, 2009. Current theory: Multifactorial, involving several pathways. • Protein accumulation: plaques & tangles • Inflammation: Unregulated activation of glia • Lipid distribution: Lipid membrane site of APP cleavage. Targets for Future Therapies • Ab ─ b-secretase inhibitors ─ -secretase inhibitors ─ Monoclonal antibodies • Tau protein • Inflammation • Insulin resistance Inhibiting neurodegeneration (clinical trials) Phase II/ Phase III 1. Inhibitors of Ab aggregation (Immunological approach; antibody directed against Ab). 2. Inhibitors of b- and g-secretase. 3. Immunological approach (antibody directed against Ab). 4. Aβ vaccination 5. Statins: HMG-CaA reductase inhibitors Inhibiting neurodegeneration (clinical trials) Phase II/ Phase III 6. Clioquinol (amoebicidal & metal chelating agent): Ab plaques bind cooper and zinc, thus removal of these metal ions promotes dissolution of the plaques. 7. NGF (Nerve growth factor): shortage of growth factors may contribute to the loss of forebrain cholinergic neurons in Alzheimer’s disease. 8. NSAIDs (esp. ibuprofen & indomethacin) reduce Ab42 formation by regulating g-secretase (unrelated to COX inhibition)