* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Trafficking of Proteins to Membranes
Silencer (genetics) wikipedia , lookup
Theories of general anaesthetic action wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Biochemistry wikipedia , lookup
Gene expression wikipedia , lookup
Cell membrane wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
SNARE (protein) wikipedia , lookup
Protein folding wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Magnesium transporter wikipedia , lookup
Interactome wikipedia , lookup
Protein structure prediction wikipedia , lookup
Protein moonlighting wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
List of types of proteins wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Protein adsorption wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
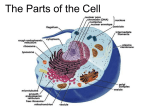
Endomembrane system wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
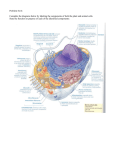
Trafficking of Proteins to Membranes 1. Protein fate is determined by N-terminal signal sequences 15-30 amino acids long. All contain ~10 hydrophobic residues. 2. When 70-80 amino acids have been polymerised during translation, and the signal sequence has emerged into the cytosol, it is recognised by the 54kDa component of the SRP. In eukaryotes, the SRP comprises 6 different proteins bound to a 300nt RNA molecule. A translational pause domain within the SRP is positioned at the interface between ribosomal subunits, and prevents elongation factor binding, slowing or stopping translation. 3. The ER membrane contains in integral membrane complex, the SRP receptor, and also a ribosome receptor. The SRP is recognised by the SRP receptor, and docking occurs. The ribosome receptor becomes a translocation tunnel, and the SRP detaches back into the cytosol. The extending, unfolded protein traverses the membrane via an aqueous pore (the Sec61 complex) in the translocator. This is mainly composed of alpha helices, and a short helix gates the complex, moving aside when a protein needs to traverse the membrane. 4. To be embedded in a membrane, the protein may contain an internal hydrophobic ‘stop-transfer’ sequence which stops translocation, leaving the protein embedded in the ER membrane. If there need to be several transmembrane domains (multipass proteins), there will be several start-transfer and stop-transfer sequences. 5. The first stage of glycosylation occurs as the protein enters the ER lumen. A precursor oligosaccharide is already assembled, attached to a dolichol lipid via highenergy phosphate – hydrolysis of this provides the activation energy for glycosylation. Glycosyl transferases add the sugar chain to the protein. 6. Once in the ER the N-terminal signal peptide is cleaved by signal peptidases. Chaperone proteins like ATP-dependent foldases (such as BiP) bind to exposed hydrophobic surfaces that should be buried in the mature protein to help correct folding. If the protein is destined to be extracellular or inside an organelle lumen, SH groups on cysteine residues are often oxidised by PDI (protein disulphide isomerase) to create strong disulphide bridges. 7. Also in the ER, covalent attachment to phospholipids or fatty acids occurs, which may attach the protein to a membrane later on. 8. When ready to leave the ER (and correct folding has been mediated by chaperones – mis-folded proteins still bound to BiP will be degraded in the cytosol by proteasomes) the proteins are packaged into COPII coated transport vesicles. These are delivered to the cis face of the Golgi apparatus. The protein passes through the Golgi towards the trans face, by repeated vesicle budding/fusion, as well as the steady maturation of cisternae as they progress from cis to trans. 9. Various types of modification occur in the Golgi. Some mannose sugars added in the ER are usually removed, before a variety of other sugars are added to the glycoprotein. The resulting oligosaccharide may be complex with branches, and account for more than 50% of the glycoprotein’s mass! 10. Proteins that need to head back to the ER are directed with a C-terminal tetrapeptide KDEL ‘retention sequence’. 11. Sorting sequences direct proteins from the trans face of the Golgi to various parts of the cell, e.g. lysosomes, peroxisomes, mitochondria, chloroplasts. A lack of sequence directs the protein to the plasma membrane/secretory pathway. Vesicles bud off the Golgi and travel to the correct location. Once the sorting process is complete, signal peptidases cleave any signal sequence. Extras Transport to Mitochondria - - Mitochondria have a double membrane, resulting in 2 compartments (matrix and inter-membrane space). Mitochondrial proteins are synthesised as mitochondrial precursor proteins, before being translocated to the mitochondria. If destined for the matrix, they have an amphipathic alpha helix signal sequence, whereas membrane/intermembrane proteins have a different signal. Proteins are translocated across the mitochondrial membranes via TOM, TIM, SAM, OXA complexes. Tim23 has Hsp70 ATPase bound, which uses ATP hydrolysis to pull proteins through the inner membrane. The precursor proteins are imported unfolded, bound to chaperones. They only fold once engaged with the TOM complex in the outer membrane. Chloroplast proteins - Also a post-translational process requiring energy, and that uses amphipathic Nterminal signal sequences that are removed after use. - Chloroplasts also have a thylakoid membrane that needs many proteins such as ATP synthase to be embedded in it. - Such proteins have two N-terminal signal sequences. The first gets them through the double membrane into the stroma. This is then cleaved, and the second is exposed, directing them into or through the thylakoid membrane. Peroxisome proteins - Unlike mitochondria/chloroplasts, peroxisomes have no DNA so all proteins are encoded in the nucleus and imported. - They contain oxidative enzymes e.g. catalase and urate oxidase. May have evolved when O2 levels first rose to use up toxic O2 and also carry out useful reactions e.g. detoxification and B-oxidation. - Peroxisome proteins are identified by a short C-terminal signal sequence of Ser-LysLeu. To import them, peroxin proteins form a translocator. Some, e.g. Pex5, accompany the imported protein all the way into the peroxisome, before being released back into the cytosol. A mutation in another peroxin, Pex2, causes Zellweger syndrome: proteins cannot be imported into peroxisomes, leaving them ‘empty’. Individuals die soon after birth. Trafficking to the nucleus - Nuclear localisation signal sequences are characterised by many positively charged lysine/arginine residues. The precise sequence varies, and can be located almost anywhere in the protein’s amino acid sequence. Thought to be exposed as loops or patches on the protein surface. - They are imported via a NPC – made up of over 30 different proteins, and a mass of 125million kDa! 500 molecules pass through per sec, and in both directions at once. - Can occur by passive diffusion for proteins <60,000 Da. Larger molecules need active transport. Large proteins or ribosomal subunits (30-40nm!) don’t need to be unfolded. - Nuclear import receptors control the process. These bind to both the protein to be transported and the NPC proteins. NPC proteins contain many phenylalanine/glycine repeats (FG repeats), to which the receptors bind. Receptorcargo complexes move through the pore by repeatedly binding/dissociating to adjacent FG repeat sequences, hopping through the NPC interior.