* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Marwa Yahia Ahmed_o
Epitranscriptome wikipedia , lookup
X-inactivation wikipedia , lookup
Genome evolution wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
Protein moonlighting wikipedia , lookup
Non-coding DNA wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Genetic engineering wikipedia , lookup
Gene desert wikipedia , lookup
DNA vaccination wikipedia , lookup
Gene expression programming wikipedia , lookup
Genome (book) wikipedia , lookup
History of genetic engineering wikipedia , lookup
Gene therapy wikipedia , lookup
Neuronal ceroid lipofuscinosis wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Gene nomenclature wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Genome editing wikipedia , lookup
Gene expression profiling wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Primary transcript wikipedia , lookup
Point mutation wikipedia , lookup
Microevolution wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Helitron (biology) wikipedia , lookup
Designer baby wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
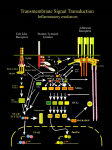
IL1RN 64 Interleukin 1 receptor antagonist The third member of the IL-1 family, IL-1Ra is a naturally occurring receptor antagonist that provides some protection against the disease-provoking effects of IL-1. It is a specific inhibitor of IL-1 activity that acts by blocking the binding of IL-1a and IL-1b to its cell-surface receptors (Merhi-Soussi et al., 2005). Interleukin-1 receptor antagonist (IL-1RN) gene Gene structure: IL-1RN gene contains four exons (nucleotide sequence that not involved in the gene transcription) and three introns (nucleotide sequence that involved in the gene transcription) that span 6.4 kilobase (Kb) of DNA. The first exon encodes a short 5'-UTR, the prepeptide portion of the precursor polypeptide and the N-terminal of mature form of IL-1Ra, while the next three exons encode the remaining part of the mature form of which the last exon encodes the 3'-untranslated region (Eisenberg et al., 1991), figure 15. 64 IL1RN 65 Figure (13): IL-1RN gene (Eisenberg et al., 1991) The first intron of the IL-1RN gene splits the 14th codon (a set of rules by which information encoded within genetic material “DNA or mRNA sequences” is translated into proteins) of the mature protein. The last two introns interrupt the coding sequence for the mature IL-1Ra polypeptide. The second intron contains perfect tandem repeats of a conserved 86-bp sequence (Ingrid et al., 2004). 65 IL1RN 66 Figure (14): The gene (Gilbert 1978) This stylistic diagram shows a gene in relation to the double helix structure of DNA and to a chromosome (right). The chromosome is X-shaped because it is dividing. Introns (sections of DNA that is not expressed in the gene product) are removed in the splicing process (after the DNA is transcribed into RNA). Only the exons encode the protein. The diagram labels a region of only 55 or so bases as a gene. In reality, most genes are hundreds of times larger. Figure (15): Exons and introns in the gene transcription (Ingrid et al., 2004). 66 IL1RN 67 Figure (16): IL-1RN gene duplication (Eisenberg et al., 1991). Product of IL-1RN gene: IL-1Ra is produced as four different isoforms, one secreted glycoprotein; secretory IL-1Ra (sIL-1Ra) which contains a classical signal sequence and is secreted, and three intracellular non-glycosylated forms having no signals sequence, remain strictly intracellular and not secreted (Nicolas et al., 2005). They are all derived from the same gene, by means of alternative first exon usage, alternative mRNA splicing and alternative translation initiation ( Merhi-Soussi et al., 2005). 67 IL1RN 68 . Figure (17): IL-1RN gene transcription (Muzio et al., 1999 ). IL-1Ra protein characteristics IL-1Ra protein shares approximately 26% a.a sequence homology with IL-1band 19% homology with IL-1a Antonis et al., 2004). IL-1Ra shows homology to the Cterminal region of both IL-1a and IL-1b. In the N-terminal region of the protein, nonsynonymous sites exist, thus providing an explanation for the biosynthetic and functional differences between IL-1Ra and the two IL-1 agonists (Eisenberg et al., 1991). 68 IL1RN 69 One of the notable differences between IL-1Ra and the two IL-1 agonist proteins lies in their mechanism of secretion from the cell. IL-1Ra appears to be a typical secreted protein, whereas both IL-1a and IL-1b are directed to the cell surface and secreted through an uncharacterized pathway involving a non leader peptidase-catalyzed proteolytic event. The secretion of IL-1Ra is apparently mediated by a classical signal sequence present at the N-terminus of the protein that is encoded in the first exon of the IL-1RN gene. Neither IL-1a nor IL-1b has such a sequence (Steven and Vera, 2000). Figure (18): Mechanism of action of IL-1Ra (Danielle and Jean-Michel, 2000). 69 IL1RN 70 IL-1Ra binds to extracellular portion of IL-1R1 without initiating intracellular signal transduction processes normally occurring after engagement of the receptor with IL-1, because it binds to site A. Despite near equal affinities to IL-1 receptors, IL-1Ra needs larger molar excess (10- to 100-fold) to inhibit the IL-1 agonistic action in vitro (Beáta, 2000). Cellular sources of IL-1Ra cytokine In general, all cell types able to produce IL-1a and/or IL-1b will also express IL1Ra. Various IL-1Ra isoforms are produced by monocytes, macrophages, neutrophils, hepatocytes, synovial and dermal fibroblasts, and several epithelial cells especially keratinocytes ( Nicolas et al., 2005). Regulation of IL-1Ra expression Expression of the various IL-1Ra isoforms is cell type and stimulus-specific. The production of IL-1Ra is stimulated by viral products, cytokines (such as IL-1a, IL-3, IL4, IL-6, IL-10, IL-13), platelet-derived growth factor and Activin A, TNF-, bacterial lipopolysaccharides and direct cellular contact with stimulated T cells, while on the contrary the synthesis and secretion of IL-1Ra can be inhibited by glucocorticoids (Kovalovsky , 1998). Production of IL-1Ra is cell specific, C-reactive protein triggers the production of IL-1 and sIL-1Ra in peripheral blood monocytes but inhibits it in alveolar macrophages. IFN-g and - modulate the production of IL-1Ra in activated human neutrophils while IFN- is a potent inducer of IL-1Ra production in monocytes and microglial cells (Nicolas et al., 2005). 70 IL1RN 71 Biological activities of IL-1Ra In normal conditions, IL-1Ra is known to be involved in health maintenance by masking coexisting IL-1 activity in tissue. The major biological role of extracellular sIL1Ra isoform is to compete with IL-1 agonists for binding to cell surface receptors, thus modulating their effect. Whereas, the intracellular IL-1Ra may be a storage form that is released upon necrotic cell death to limit inflammation caused by cell debris (Gabay et al., 2001). It performs important regulatory roles within cells as it functions as a unique intracellular inhibitor that alters IL-1-inducible gene expression, destabilizes and reduces the half-life IL-1a mRNAs, and attenuates IL-1 responses at a point downstream of the initial IL-1/IL-1 receptor interaction ( Arend et al., 1998). IL-1Ra production in women and men IL-1Ra is normally present in the circulation of healthy persons, whereas IL-1a and IL-1b are not typically detectable in the absence of disease or autoimmunity. A plausible explanation for this is that the circulating IL-1Ra may function to prevent the unnecessary activation of proinflammatory immunity in response to minor nonpathogenic stimuli. The production of IL-1Ra, as well as IL-1, appears to differ between men and women. The in vitro production of IL- 1RA from isolated monocytes was significantly higher in women than in men (Khosla et al., 1994). Furthermore, monocytes collected during the proliferative stage of the menstrual cycle released 2–3-fold greater levels of IL-1Ra than did monocytes obtained from women in the luteal phase of the menstrual cycle. Similarly, IL-1Ra levels in cervical mucus were lowest during the luteal phase in ovulating women. This highlights the 71 IL1RN 72 involvement of estrogen levels in regulating the IL-1 system. Primarily on the basis of these data, it has been suggested that the regulation of IL-1Ra and IL-1 production may be fundamentally different in women from in men. IL-1Ra levels were shown to also be significantly higher in healthy 79-year-olds than in healthy 39-year-olds. The significance of this observation to the decline in immunity with increasing age remains to be evaluated. Interestingly, levels of IL-1Ra in amniotic fluids and urine of newborns were also shown to be significantly higher in female neonates than in males (Arend et al., 1998). IL-1Ra production in healthy and disease states IL-1Ra is normally present in the circulation of healthy persons at low levels, whereas IL-1a and IL-1b are not typically detectable in the absence of disease. This circulating IL-1Ra may function to prevent the unnecessary activation of proinflammatory immunity in response to minor nonpathogenic stimuli. IL-1Ra levels are higher in women than men especially among elder individuals. Some people produce higher levels of IL-1 than other people, and this high production of IL-1 tend to run in families (Cullup et al., 2004). The circulating blood levels of sIL-1Ra are elevated in several infectious, autoimmune, chronic inflammatory diseases, neoplastic diseases, and following surgery. In addition, the levels of IL-1Ra are locally enhanced within the joints in acute and chronic disorders such as RA, OA, Lyme arthritis, and nonperforating Crohn’s disease (Smith et al., 2004). 72 IL1RN 73 Balance between IL-1 family members The mechanism of action and relationship between IL-1 agonists and their antagonists has several levels. In the synthesis pathway, production of agonists and antagonists is regulated at the level of the pre-transcriptional process, transcription, posttrancriptional mRNA splicing, mRNA stability, translation and processing. IL-1 is an auto-regulating protein, inducing its own expression in human and in vitro. This autoinduction can be inhibited or up-regulated by IL-1Ra, thus suggesting that IL-1Ra is a negative and a positive regulator of IL-1 agonist production (Beáta , 2000). Other cytokines may also contribute to the balance between the production of IL-1 agonists and IL-1Ra in disease. For example, IL-4, TGF-, and IL-10 increase the production of IL-1Ra but at the same time decrease the production of IL-1a (Dinarello and Wolff , 1993). The overall bioactivity of IL-1 appears to be determined by the relative concentrations of IL-1 and IL-1Ra, rather than by the absolute concentration of IL-1 alone. In vitro experiments have revealed that an excess of 10-100 times the amount of IL-1Ra is necessary to inhibit IL-1 activity whereas, in vivo, studies showed that 1002000 times more IL-1Ra is needed (Pelletier et al., 1995). 73 IL1RN 74 IL-1RN Gene polymorphisms It is becoming increasingly clear, as the DNA sequences of the human genome are being revealed, that many genes are polymorphic i.e., variable in DNA sequence. In coding or non-coding regions of a specific gene, there may be either a single base pair substitution of one nucleotide for another; figure 19, or a variable number of repeats of a short, repetitive DNA sequence. Short tandem repeats (STRs) are short sequences of DNA, normally of length 2-5 base pairs, that are repeated numerous times in a head-tail manner. The polymorphisms in STRs are due to the different number of copies of the repeat element that can occur in a population of individuals ( Butler, 2006) Figure (19): DNA molecule 1 differs from DNA molecule 2 at a single base-pair location (a C/T polymorphism) (Barreiro et al., 2008). 74 IL1RN 75 These variations may influence the rate of gene transcription, the stability of the messenger RNA, or the quantity and activity of the resulting protein. Thus, the susceptibility or severity of a number of disorders will be influenced by possession of specific alleles of polymorphic genes. One polymorphic gene that has received a great deal of research interest is the gene coding IL-1RN. This gene is located on chromosome 2 in close proximity to the genes coding for IL-1a and L-1b (Steinkasserer et al., 1992). IL-1a and IL-1b are major inducers of proinflammatory immune responses. They both bind to the same IL-1 receptor on the surface of a variety of cells and initiate a cascade of events leading to recruitment and activation of macrophages and neutrophils, vascular dilation and fever, and a potent proinflammatory immune response (Dinarello, 1988). The central role of the IL-1 system is protection against many different insults, ranging from microbial colonization to infection to malignant transformation. IL-1Ra also binds to the same IL-1 receptor but does not initiate signal transduction. IL-1Ra is thus a competitive inhibitor of IL- 1 bioactivity. The relative levels of IL-1Ra and IL-1 at an inflammatory site will thus determine whether a proinflammatory response will be initiated and persist or will be terminated (McIntyre et al., 1991). Typically, the concentration of IL-1Ra increases late during the course of an inflammatory event so that an induced acute inflammation can terminate and does not become chronic and damage healthy cells (Granowitz et al., 1991). 75 IL1RN 76 Influence of gene polymorphisms on IL-1 expression Cytokines and cytokine receptors are generally highly conserved in their coding regions. Polymorphisms within the exons directly affect the protein structure and possibly result in modulation of protein production and/or alteration of protein function (Moos et al., 2000). . Figure (20): Diagram of the "typical" eukaryotic protein-coding gene (Cech , 1986). Promoters and enhancers determine what portions of the DNA will be transcribed into the precursor mRNA (pre-mRNA). The pre-mRNA is then spliced into messenger RNA (mRNA) which is later translated into protein. 76 IL1RN 77 bp= base pair. Figure (21): Polymorphism in IL-1 gene complex (Robert et al., 2003) In vitro and in vivo secretion of IL-1b and IL-1Ra exhibits inter-individual variability associated with gene polymorphisms. IL-1b +3953 and -511 single base substitution polymorphisms participate in the regulation of IL-1Ra in vivo production. IL-1RN*2 genotype increases IL-1Ra levels in vivo in healthy population only in the presence of allele 2 of IL-1b -511. Allele 2 of IL-1b-511 and IL-1RN*2 are considered to 77 IL1RN 78 represent a “high secretor” phenotype leading to increased proinflammatory activity in autoimmune and infectious diseases (Wei-Xing et al., 2005). Several studies reported significantly higher blood levels of IL1Ra protein were associated with allele T at one polymorphism (rs4251961) that is part of the broader haplotype marked by the CTA alleles. In addition, allele C at rs419598 of the CTA haplotype has been associated with increased peripheral blood mononuclear cell expression of IL1Ra and decreased risk for osteolysis after total hip arthroplasty (Gordon et al., 2008). The lower synovial fluid (SF) levels of IL10, IL1b and IL6 in the presence of the IL1RN CTA haplotype are consistent with lower IL1 biological activity, as would be expected in individuals with increased IL1Ra expression and a lower level of activation of local cytokine-producing cells. Although no simple relationship was seen between the SF levels of IL1Ra and carriage of the IL1RN CTA haplotype in all patients, this relationship was evident in patients who were not in the highest tertile of IL10 levels. IL1Ra expression is directly activated by IL10 in both monocytes and neutrophils (Cassatella et al., 2005). The finding of a higher level of ILRa in SF samples from patients carrying the CTA haplotype only in the lower two tertiles of IL10 protein is consistent with a genetic effect that may be evident only under submaximal activation conditions. For example, if one assumes near-maximal activation of IL1Ra when IL10 is strongly elevated, as would be expected in tertile 3 of IL10 levels, a genotype effect on IL1ra expression may be less evident than at submaximal levels of IL10 (Ryder et al., 2008). 78 IL1RN 79 Thus, the susceptibility or severity of a number of disorders will be influenced by possession of specific alleles of polymorphic genes. Environmental exposures can also be important determinants of whether a specific allele contributes to disease risk (Beáta, 2000). 79