* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download L16v03-growthApop.stamped_doc
Survey
Document related concepts
Protein phosphorylation wikipedia , lookup
Cell nucleus wikipedia , lookup
Biochemical switches in the cell cycle wikipedia , lookup
Cell membrane wikipedia , lookup
Cell encapsulation wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell culture wikipedia , lookup
Endomembrane system wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell growth wikipedia , lookup
Cytokinesis wikipedia , lookup
Programmed cell death wikipedia , lookup
Signal transduction wikipedia , lookup
Transcript
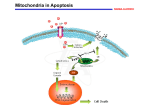
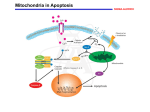
L16v03-growthApop [00:00:00.00] [00:00:01.22] SPEAKER 1: I will conclude in this video our discussion of the cell cycle with three topics. We'll talk about how mitogens stimulate cell division. We'll talk about that as being different from growth factors, which promote growth, promote cell survival, and prevent cell death. And then we'll conclude talking about apoptosis, which is the mechanism which cells use when they decide not to survive, not to grow any longer, but to commit cellular death. Which might be part of a developmental program in the formation of organs. Or as a result of something having gone wrong. [00:00:38.00] On the slide we differentiate between these categories a little bit better, specifying mitogens as something that causes specifically cell division and cytokinesis. It doesn't matter how large or small the cell is, let's divide. [00:00:53.89] Growth factors are primarily about increasing cell mass. It's not dividing cells, but it's producing protein, producing lipids, producing the molecules that are needed biosynthetically to make a cell larger. [00:01:07.25] And then there are finally things specific as survival factors are required for nearly all eukaryotic cells for continued survival. Many eukaryotic cells use connections with the extracellular matrix as a signaling factor to continue to survive. When survival factors are absent, that's when the cells will trigger apoptosis and commit cell suicide. [00:01:34.87] So cell division, accumulation of mass, and avoidance of death. [00:01:43.20] So most mitogens are extracellular ligands that bind to cell surface receptors. They start of signalling pathway which will show part of its effect by acting on a very important tumor suppressive protein called RB or retinoblastoma. The name retinoblastoma is given to it because, if it is mutated and not functioning properly, people tend to develop tumors of the eye. Its normal function is to put the brakes on cell division by inactivating transcription factors that are necessary for progression into the S phase. [00:02:22.58] When a mitogen binds, it'll start a signaling cascade where a kinase will eventually phosphorylate the retinoblastoma protein, which inactivates it. This allows the transcription factors to bind transcribed genes that's going to promote the transition to the S phase and allow the replication of your DNA. [00:02:46.05] So here is an example where we've talked that most of the time people think of the phosphorylated protein as being active. In this case retinoblastoma is phosphorylated to inactivate it. , However it happens to be an inhibitor. So when retinoblastoma is phosphorylated, then you have transcription of the genes. So it's sort of like multiple negative 1 twice. By inhibiting an inhibitor, you are activating a pathway. [00:03:16.26] Some pathways serve both growth signals and mitogen type signals. As we can see here, there are a lot of familiar players. We have the receptor tyrosine kinases, which are activated by ligand-induced dimerization. This is going to phosphorylate inositol phosphorolipids. And this is going to activate a pathway called jointly AKT/mTOR. And this is the primary pathway that's going to stimulate protein synthesis, the accumulation of mass, and cell growth. [00:03:52.25] This is in another pathway that is very frequently hijacked in cancers, causing the cell to grow even though it's not appropriate for that particular developmental state. These are primarily kinases which are regulating these processes, so we have protein phosphorylation. And here we have an example of a series of activations to achieve a desired phenotype. [00:04:18.48] And here we see the inhibition of an inhibitor, again to stimulate a particular phenotypic response. [00:04:25.03] On this slide we just want to highlight the difference, make it very clear, the difference between cell growth and cell division. On the left you see a neuron, that's a single cell. On the right you see a lymphocyte, that's one white blood cell. These are drawn roughly to scale in this case. And clearly the neuron has accumulated a lot more mass. There's many times more protein lipids and every other biomolecule associated with it, other than the fact that the two cells have the same amount of DNA. [00:04:54.07] But as you know with respect to cell division, neurons are generally, in adults, not dividing. Whereas lymphocytes are some of the most rapidly dividing cells in your body. [00:05:06.20] On this slide we see an example of survival factors which are released, in this case, by target cells in red dots here. The amount of survival factor released by these target cells can only support a limited number of neurons. In fact, in a number of different scenarios you want to have one nerve cell monitoring one target cell, or responding to or controlling one target cell, that redundant information or control of a cell is unneeded. So while it is stochastic in terms of which one of these two cells might win out or survive, the fact is only one of the two neurons will survive for each target cell. [00:05:48.96] As with the visual system, the amount of survival factors released by any particular target cell is dependent on activity. And you may have heard about the studies where, if a visual system is not used during key development phases, those neurons will all die and they will never be able to regenerate. Leaving that organism functionally blind. So the survival factor production of target cells can be activity dependent. [00:06:17.74] So now we'd like to say a few words about apoptosis., which I've sometimes referred to as planned cellular suicide. And these first examples are highlighting the fact that we want to show that this is part of the normal plan. This is not happening by accident. [00:06:34.06] Here you see a developing limb, which initially starts as a single bud. And then you can see there's some bright spots here. And these are cells that are immunofluorescing, that they are undergoing apoptosis. They are purposely have triggered pathways, which is going to cause their cell death. By doing so you're going to create furrows between digits so that a single smooth limb bud can now develop into a five projectile or five appendage limb. [00:07:10.53] And again, this is not something that has to happen early in development. This also is what occurs how tadpoles will lose their tail when they're no longer dependent upon, so dependent upon swimming. Of course, apoptosis is also used in response to massive cell damage which is not pre-planned, but it is the cell's reserved way of dealing with insurmountable problems. [00:07:40.89] Apoptosis, which is programmed cell death, or orderly cell death, is contrasted here with necrosis, another form of cell death. This is the equivalent of dying by traumatic injury. Whether there's a blunt force or starvation, in the case of where a blocked blood vessel would not be supplying this. Or at the center of a tumor, where there's simply not enough blood ever being delivered to support the increase in cell mass. [00:08:18.28] This is a nasty event. And this will provoke an inflammatory response in your body, which comes with its own benefits and risks. Immune cells start arriving. They start releasing all sorts of cytokines. And it can make it a pretty stressful cellular situation. [00:08:38.54] Apoptosis, on the other hand, precedes in an orderly manner. DNA is shredded, it's cleaved into pieces. Ribosomes are started to-- proteins are starting to be cleaved. Organelles are destroyed. You start to see the appearance of blebbing vacuoles. And the cell will start to slowly shrink from the inside. [00:08:59.09] Eventually the cell will be engulfed by a cell of the immune system, a phagocytic cell. But not in a pro-inflammatory way. Again, very orderly disposal of cell constituents. [00:09:15.14] The molecular machinery, which produces apoptosis relies on a type of protein, a large protein family called caspases. There are a series of them that are activated in turn to produce the apoptotic results in a cell. Most of these caspases exist in the cells as procaspases. These are inactive forms of the caspases. [00:09:41.55] Once there is an activation by an apoptotic signal, this procaspase will cleave into the active form. And then as a protease it will activate other procaspases in a sequential feed forward mechanism, eventually activating proteases that will chew just about every protein part of the cell. [00:10:02.87] The point of the return is at the initial signaling, when the apoptotic mechanism is initiated. There are two particular types of that initiation, which we'll see in future slides. [00:10:19.86] The two pathways that activate apoptosis are the extrinsic and the intrinsic pathway. Quantitatively the intrinsic pathway, which we'll see next, is more predominant. But the extrinsic pathway is very important too. [00:10:36.58] This is a pathway where the signal comes extracellularly. Here's the target cell. It is triggered by the immune system. and one area where this will occur is if the immune system detects that this particular cell is invaded by viruses. If viruses have infected this cell, there will be a molecular change on the surface which will alert the immune system to destroy the cell. [00:11:08.17] The immune cell that does it is called a natural killer lymphocyte. And on its surface they have something called the fast ligand. The fast ligand can bind to the fast death receptor, which is not universally expressed in all tissues. But can be stimulated to be expressed in most tissues by certain pro-inflammatory molecules. Again, if a virus has infected a cell, these pro-inflammatory molecules are likely to be around. So many of those cells in that environment will express a fast death receptor. [00:11:41.83] However, it still remains for a killer lymphocyte to recognize this fast death receptor. And by binding here, you're going to activate a number of these caspase proteins, which then can trigger a sequence of activation events of various procaspases. And then you're going to undergo the normal apoptotic cellular pathway. [00:12:07.75] This is referred to as the intrinsic pathway of apoptosis, and perhaps the more numerically important. A key organelle in the intrinsic pathway is going to be the mitochondrion. And surprisingly, the protein cytochrome c, which is abundant in the inner mitochondrial membrane, is going to play a major role. [00:12:30.87] You'll remember cytochrome c from the electron transport chain in oxidative phosphorylation. This is one of the proteins that shuttled electrons between cytochrome bc1 and cytochchrome c oxidase, two protein complexes which transfer electrons and pump protons. Cytochrome c did not pump protons. It just shuttled-- it has one of the lipid tails, so it's bound to the surface of the membrane. And it shuttled electrons from the second proton pumping complex to the final proton pumping complex. [00:13:04.50] And that's its major role. And it exists here in the mitochondria. However, if the cell detects a signal to induce apoptosis, this could be the result of binding a ligand to promote apoptosis. It could be as a result of no longer receiving a survival factor. It could be as a result of a lot of DNA damage. [00:13:30.02] For whatever reason, if the cell is intent on initiating apoptosis, what it will do is it will create pores in the outer mitochondrial membrane. It will destroy the linkage between the lipid that tethers cytochrome c, and cytochrome c will spill out into the cytosol. The cell detects that cytochrome c is not a protein that's supposed to be out in the cytosol. It is only supposed to be in the mitochondrion. [00:14:02.37] And so it's just the fact that cytochrome c is in the cytosol that's going to trigger the activation of procaspases by cleavage, so that you get activated caspases. And now you're going to get proteins that will start degrading nucleic acids, degrading all the protein machinery of the cell. [00:14:21.13] So this is really a dual functional use for cytochrome c. In normal respiration it's an electron transfer protein. But here it's been used as a signal protein to alert the fact that something has tipped the balance of cell survival, and it is time to commit apoptosis. [00:14:41.15] Now the channel which allow cytochrome c to escape for the mitochondria is formed by proteins called backs or back. And one way that the cell, and we'll see this on the next slide, one way the cell will prevent the formation of these proteins is a protein called BCL-2, which will ensure that these pores stay closed. [00:15:01.24] The final point to make is when cytochrome c hits the cytosol or cytoplasm, it doesn't really matter where it came from. Most of the time the salt comes from the mitochondria. But if you were to artificially inject the cytochrome c protein into a cytoplasm, that would have the same effect. It's simply the presence of cytochrome c where it should not be that's going to trigger apoptosis. [00:15:27.46] And here we see that survival factors, one way that they allow cellular survival, is by a cellular cascade which will result in a series of phosphorylation events, activating a transcription factor, which is going to produce the BCL-2 RNA, the BCL-2 protein. The BCL-2 protein is unfolded, transported into the mitochondria, and prevents the pore from forming on mitochondria, and thus ensuring that cytochrome c stays within the mitochondrial matrix. [00:15:59.80] In the last slide, I'd just like to draw a general analogy between sort of a generic signaling pathway and what we've seen here with the intrinsic pathway of mitochondria. This is sort of what people think about, a signalling pathway with an extracellular ligand binding to a receptor in the plasma membrane. A series of phosphorylation events, or production of small molecules to transmit a signal towards effector proteins, either changing protein activity, changing the transcription of certain genes in response to the extracellular signal, or altering cell phenotypes in some manner. [00:16:36.55] This is in every way an analogous pathway. The receptor would be some apoptotic stimuli which corresponds to essentially the presence of a ligand. This apoptotic stimuli could again be ultraviolet damage to DNA and broken pieces of DNA. That might be what signals apoptosis to occur. [00:17:00.06] Then we have a series of molecular events. The formation of pores, the release of cytochrome c, the activation of caspases towards a particular phenotypic effect. So you don't necessarily have to think of signaling pathways as always originating on the outside of the cell to produce a result on the inside of the cell. [00:17:21.91] These signaling pathways can be entirely contained within the cell. Although in the example I gave, you might argue that the UV photon which causes the cell death is an external signal from the environment. And of course you'd be right about that. Nevertheless, thanks for listening.