* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Protein Structures
Phosphorylation wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Magnesium transporter wikipedia , lookup
List of types of proteins wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Protein moonlighting wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein domain wikipedia , lookup
Homology modeling wikipedia , lookup
Protein folding wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
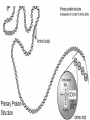
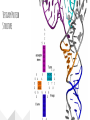
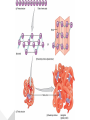
Protein Structures Explanation of Protein Structures Check out this link for more detail. Rules to remember... The sequence of the nucleotide determines the amino acid. The amino acid sequence determines the protein shape. The protein shape determines it function. Primary Protein Structure Primary Primary protein structures refer to the order in which amino acids link together. Proteins combine to form a chain of amino acids. The amino acids include an alpha carbon, which is bonded to a Hydrogen atom, a carboxyl group and an amino group. Amino acid sequences of a protein are determined by the information found within the cellular genetic code. The order of amino acids within the polypeptide chain is completely unique, and is specifically linked to a protein. When amino acids are changed within a sequence, a gene mutation occurs, which often results in a protein that is non-functioning. Secondary Secondary protein structures occur when polypeptide chains coil or fold, giving them a 3D shape. Alpha(α)Helix structures resemble a coiled spring, and result from hydrogen bonding within the peptide chain. Beta Pleated structures seem to be folded, or pleated and form when hydrogen bonds between adjacent polypeptide units Check out this link to learn more about secondary protein structures. Tertiary Protein Structure Tertiary Tertiary protein structures deal with the 3D shape of a polypeptide chain. There are multiple bonds/forces that contribute to the protein’s ability to remain in tact. Hydrophobic interactions often play a key role in maintaining a protein’s shape. “R” groups in amino acids are either hydrophobic or hydrophilic and will seek aquatic or non-aquatic environments accordingly, which determines their location within the protein. Hydrogen bonds facilitate stabilization within the proteins based on the shape established by the hydrophobic interactions. Ionic bonding causes folding between positively and negatively charged R groups. Check out this link to learn more about tertiary protein structures. Quaternary Structure Quaternary Quaternary structures deal with the structures that between multiple polypeptide chains. Each chain is referred to as a subunit. Structures may consist of multiple polypeptide chains that may either be the same subunit, or different subunits. Click this link to learn more about quaternary protein structures. Why do they change structures? What can cause a protein structure to change? Changes in pH Changes in salt concentration Changes in temperature Presence of reducing agents Where do you find these structures? How has environmental changes impacted our need for different types of proteins?