* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Proteins have a higher order of folding known as tertiary structure
List of types of proteins wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Western blot wikipedia , lookup
Structural alignment wikipedia , lookup
Circular dichroism wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Homology modeling wikipedia , lookup
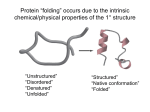
Protein folding wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein domain wikipedia , lookup
Metalloprotein wikipedia , lookup
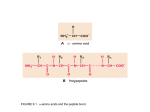
Proteins have a higher order of folding known as tertiary structure. The tertiary structure is a description of the way the whole chain (including the secondary structural features) folds itself into a 3D shape. The structure is held in place by interactions between the side chains (R groups) of the amino acids which made up the primary structure. o o o Amino acids such as aspartic acid or glutamic acid contain a carboxylic acid in their side chain. This can lose a proton to give a negatively charged COO-. Lysine, for example, contains a positively charged NH3+ group. If the protein folds in a suitable manner, these oppositely charged groups are able to form an ionic bond. Aspartic acid residue Ionic bond Lysine residue Polypeptide backbone Serine residue H-bond o o o Asparagine residue o o You should know that hydrogen bonds between the polypeptide backbone are responsible for maintaining a protein’s secondary structure. However, many amino acids have side chains which contain a hydrogen atom attached to an oxygen or nitrogen, and can therefore form hydrogen bonds. For example, serine contains an OH group while asparagine contains an amide. The diagram shows how these R groups can form a hydrogen bond. Several amino acids (including leucine, valine, and phenylalanine) have non-polar hydrophobic side chains which contain just carbon and hydrogen atoms. Two of these groups brought close to each other will both repel water and other polar groups, resulting in an overall attraction to each other. Hydrophobic stacking Phenylalanine residues Disulfide bridge o Cysteine residues If two cysteine residues are near to each other, they can react to form a covalent bond known as a disulfide bridge. The quaternary structure of a protein is a way of describing how multiple polypeptide subunits come together and arrange themselves. The interactions which hold the structure in place are the same as those for tertiary structure – however now these interactions are inter-chain (between two different chains) rather than intra-chain (between residues of the same chain). Some quaternary structures are made up of two or more identical chains. For example, HIV Protease (PDBe entry 4je2) exists as a dimer – two subunits of the same protein. Chain A (shown in blue) and chain B (shown in green) are identical – both have exactly the same primary structure (amino acid sequence). The two chains brought together give the protein its quaternary structure. Other quaternary structures are made up of two or more non-identical chains. An example of this type of structure is haemoglobin (PDBe entry 2m6z). There is a pair of α-subunits (shown in blue) and a pair of β-subunits (shown in yellow). The overall arrangement of chains is roughly tetrahedral. Each subunit binds a heme ligand which contains an iron ion (Fe2+). These Fe2+ ions allow oxygen binding, so haemoglobin can carry out its function of oxygen transport. Produced by Lucy Jakubecz at Newcastle University as part of an MChem project.









![Proteins2[1]](http://s1.studyres.com/store/data/008291804_1-fc4b593e0423ea377f021b9f7071accd-150x150.png)