* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download proteinskubalova
Paracrine signalling wikipedia , lookup
Signal transduction wikipedia , lookup
Gene expression wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Expression vector wikipedia , lookup
Magnesium transporter wikipedia , lookup
Point mutation wikipedia , lookup
Peptide synthesis wikipedia , lookup
Interactome wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Homology modeling wikipedia , lookup
Genetic code wikipedia , lookup
Biosynthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Protein purification wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Western blot wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Two-hybrid screening wikipedia , lookup
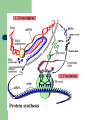
Proteins Lucie Kubalová A protein (in Greek πρωτεϊνη = first element) is a complex, high molecular weight organic compound that consists of amino acids joined by peptide bonds. is essential to the structure and function of all living cells and viruses. Many proteins are enzymes or subunits of enzymes. A peptide bond is a chemical bond formed between two molecules when the carboxyl group of one molecule reacts with the amino group of the other molecule, releasing a molecule of water (H2O). This is a dehydration synthesis reaction, and usually occurs between amino acids. Primary Structure A protein can be considered to have primary, secondary, tertiary, and quaternary structures. primary structure: the linear amino acid sequence of a protein Secondary regular (pravidelný) repeating structures arising (vznikají) when hydrogen bonds between the peptide backbone amide hydrogens and carbonyl oxygens occur at regular intervals within a given linear sequence (strand) of a protein (as in the alpha helix) or between two adjacent strands (as in beta sheets and reverse turns) Tertiary the overall three dimensional shape of a protein, often represented by a backbone trace Quaternary oligomeric structure of a multisubunit protein in which separate proteins chains associate to form dimers, trimers, tetramers, and other oligomers. The different chains in the oligomers may be the same protein (homooligomers) or a combination of different protein chains (heteroliogomers). The different chains within the oligomer may be held together by noncovalent intermolecular forces or may also contain covalent interchain disulfides. held(hold) = držet Protein nutrition in humans proteins come in two forms: complete proteins contain all eight of the amino acids (threonine, valine, tryptophan, isoleucine, leucine, lysine, phenylalanine, and methionine) that humans cannot produce themselves, while incomplete proteins lack or contain only a very small proportion of one or more Humans' bodies can make use of all the amino acids they extract from food for synthesizing new proteins, but the inessential ones themselves need not be supplied by the diet, because our cells can make them ourselves. proportion = podíl supply = zásobovat References: http://en.wikipedia.org http://www.healthepic.com http://employees.csbsju.edu Thank you very much for your kind attention