* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Protein structure
Survey
Document related concepts
Transcript
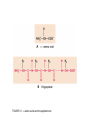
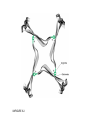
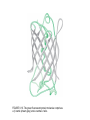
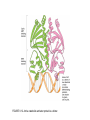
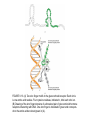
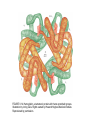
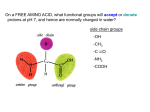
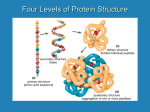
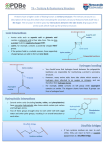
FIGURE 9.1. -amino acids and the peptide bond. FIGURE 9.2. The genetic code. Amino acid side chains are shown in alphabetical order together with the three- and one-letter amino acid abbreviations. Hydrophilic side chains are shown in green, hydrophobic side chains in black. Other important characteristics of each side chains are noted. To the right of each amino acid we show the corresponding mRNA codons. FIGURE 9.3. Histidine and cysteine have pK values in the physiological range. FIGURE 9.4. Oxidation of adjoining cysteine residues produces a disulfide bond. Proteolysis of the polypeptide releases cystine. UNFIGURE 9.1. UNFIGURE 9.2. FIGURE 9.5. Six amino acid side chains can be phosphorylated. FIGURE 9.6. Drawing of the platelet-derived growth factor receptor. FIGURE 9.7. Lysozyme: (A) Linear map. (B) Space-filling model in which carbon atoms appear gray, oxygen red, nitrogen blue and hydrogen white. None of the sulfur atoms are visible on the surface. (C) Backbone representation. (D) Cartoon. Panel B uses data from Wang et al. (2007) Acta Crystallogr. Sect. D 63, p. 1254 viewed via the Research Collaboratory for Structural Bioinformatics (www.rcsb.org) database. FIGURE 9.8. The helix. In B and C the carbon atoms of the backbone are black, with carbons so marked. Peptide bond nitrogens are deep blue and their attached hydrogens are white. Peptide bond oxygens are red. Green spheres represent the amino acid side chains. D shows only the backbone with all its atoms shown gray, plus the side chains in green. UNFIGURE 9.3. FIGURE 9.9. sheets. FIGURE 9.10. The green fluorescent protein molecule comprises a barrel (shown gray) and a central helix. FIGURE 9.11. Calmodulin is composed of two very similar domains. FIGURE 9.12. Active catabolite activator protein is a dimer. FIGURE 9.13. (A) Two zinc finger motifs in the glucocorticoid receptor. Each circle is one amino acid residue. Four cysteine residues, indicated c, bind each zinc ion. (B) Drawing of the zinc finger domains of a dimerized pair of glucocorticoid hormone receptors interacting with DNA. One zinc finger is indicated in green and corresponds to the amino acids colored green in (A). FIGURE 9.14. Hemoglobin, a tetrameric protein with heme prosthetic groups. Illustration by Irving Geis. Rights owned by Howard Hughes Medical Institute. Reproduced by permission. FIGURE 9.15. The iron-containing prosthetic group heme in the form in which it is found in oxygenated hemoglobin. The oxygen molecule is at the top. From D. Voet and J. D. Voet, Biochemistry , 2nd ed., p. 216. © 1995 John Wiley & Sons, Inc. Used by permission of John Wiley & Sons, Inc. UNFIGURE 9.4.