* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Mechanics of the Cvtoskeleton
Survey
Document related concepts
Biochemical switches in the cell cycle wikipedia , lookup
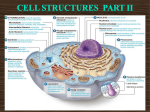
Cell nucleus wikipedia , lookup
Cell membrane wikipedia , lookup
Tissue engineering wikipedia , lookup
Signal transduction wikipedia , lookup
Endomembrane system wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cellular differentiation wikipedia , lookup
Programmed cell death wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell culture wikipedia , lookup
Cell growth wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cytoplasmic streaming wikipedia , lookup
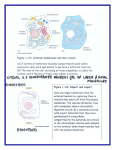
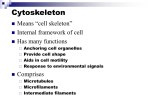
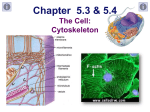
Microtubule wikipedia , lookup
Transcript
. :r..:-.':i and J.W.C. Dunlop :4.. :'l - ji. . : --'i(JIl : : \ ) r g r n i s m s .P r i n c e t o n Mechanicsof the Cvtoskeleton l : : : - : : i . . 1 1n r i c r o t u b u l e s :c l o s e Peter Nick .-::-- .-' ,rl lignin on the selfn , '.. , ' : , i Ll ru \ . C e l l u l o s e l 4 : , t : . : : : , , . l r s e r n b l !o f c e l l u l o s e / >.rj.: ,; . t9 l_50.+ :. : -i : - J i l u l o s ea n d x y l o g l u c a n . . . , i i . .P l a n tP h y s i o l I 2 l : : r : . : - . . ; r . r e t i o nu s i n g c e l l u l o s e / ' " :- . ( . I ( . r i l i c a t i o no f p e c t i c . - l c r i . l \ ea c l i o n , m a t r i x i ,::i:' .,:r.riiclgrowth-response. , - ^ j ; r . : . \ . L n dY o u n g ' s m o d u l u s . . . :\ , . I : i h n o l 3 l : l 3 l - l 4 l Abstract This chapter summarizesevidencefor a cytoskeletalfunction in tensegral integrationon both the organismaland the cellular levels.The plant cytoskeleton consistsof two major elements,microtubulesand actin filaments.The spatial organization of these elements is highly dynamic and changes fundamentally during the cell cycle, with conspicuouseffects on the predicted stress-strain patterns.In interphasecells, microtubule bundlesare thought to control the direction of cellulose depositionand thus to reinforce the axiality of cell growth. By microtubule-actinlinkers such as the novel classof plant-specifickinesins with a calponin-homologydomain, the rigid microtubulesand the flexible actin bundles can be integratedinto a systemendowedwith mechanicaltensegrity.Becausethe plant cytoskeletonis relieved of the load-bearingtask it fulfils in the non-walled animal cells, it has adoptedsensoryfunctions.Stretch-inducedchangesof protein conformation and stretch-activatedion channelsseem to act in concert with the cytoskeleton,which acts either as a stress-focussing susceptorof mechanicalforce uponmechanosensitive ion channelsor as a primary sensorthat transducesmechanical force into differential growth of microtubule pl,.:sends. This cytoskeletal tensegrity sensor is used both to integrate the growth of individual cells with mechanicalload oftissues and organsand as an intracelluliu sensorusedto control holistic propertiesof a cell such as organellepositioning.The distinct nonlinearity of microtubulesin particular rendersthem an ideal tool for self-organizationin responseto mechanicalinput from the exterior. P. Nick Botanical Institute and Center of Functional Nanostructures, Karlsruhe Institute of Technolosv. Kaiserstr. 2,'7 6128 Karlsruhe, Germany e-mail: [email protected] P. Wojtaszek(ed.1,MechanicalIntegrationof Plant Cellsand Plants, Signalingand Communicationin Planrs9, DOI 10.1007/918-3-642-19091-9*3 Berlin Heidelberg201I e Springer-Verlag 5,1 P. Nick Prologue:The SensitiveCytoskeletonor the Hidden Face of Plant Tensegrity Even before Ledbetterand Porter (1963) described"microtubules" in plant cells, which they hadobservedby transmissionelectronmicroscopy.the plantcytoskeleton was predictedto exist on the basisof biomechanicalconsiderations. It was Paul Greenwho concludedfrom their geometrythat growing plantcellsrepafiitiongrowth by an unknown"reinforcementmechanism"from the spontaneously preferredlateral expansionin favour of elongation.and he predictedthat the cell can establishand maintainthe mechanicalanisotropyof cell walls throughan as-yet-unknownlattice of tubularelementsthat are orientedin an orderedfashion(Green 1962).Thus.ever sinceits discovery,the plant cytoskeletonhas beenintimately linked with mechanical aspectsof morphogenesis,and this chapterwill thereforenot follow the usual approachto describethe plant cytoskeletontiom its molecularbasisand then derive structuresand functions.It will ratherassumea view of the cytoskeletonthat derives from its function in mechanicalintegrationof cell and organmorphogenesis. As a consequence of their photosynthetic lif'estyle,plantsincreasetheir surface by folding outwards,producinga considerabledegreeof mechanicalload. As long as they remainedaquatic,this load was partiallyrelievedby buoyancy,allowing considerable body sizesevenfor fäirly simplearchitectures. However,when plants began to move into terrestrial habitats"they had to develop flexible yet robust mechanicalsupports.The inventionof vasculature-based modules.the so-called telontes(Zimmermann1965),was the decisivefactorfbr the evolutionarysuccess of the cormophyticlandplants. Mechanicalload shapedplantarchitecture down to the cellularlevel.Plantcells areendowedwith a rigid cell wall, and this afl'eclscell divisionand cell expansion both specilicallyand fundamentally(see also chapter"Micromechanicsof Cell Walls"). The depositionof a new crosswall will definethe pattemsof mechanical strain that, during subsequentcell expansion,will guide the complex inteplay betweenthe expandingprotoplastand the yielding cell wall. It is even possible to describethe shapeof individual cells in a plant tissueas a manifestationof minimalmechanicaltension(Thompson1959),emphasizing the stronginfluenceof mechanicalload on plantdevelopment. Whenplantsarechallengedby mechanicalstimuli.theyrespondby changingthe architecture, which will allocateload-bearingelements(vesselsand fibreson the organ level, cellulosemicrofibrilsand lignin incrustationson the cellular level) guided by the imposedfield of forces.An impressiveexampleis the formation of tensionand compressionwood (for a recenlreview, see Funada2008). This architecturalresponseensuresthat mechanicalstrainsare balancedin an optimal fashionfor minimal investmentof energyand biomatter.Moreover,this mechanical balanceis continuouslyadjustedto the curent environment- a must in a system endowedwith open morphogenesis. where the Bauplon is continually extendedby additionof newly formedorgans(seealsochapter"MechanicalForceResponses ol P l a n tC e l l sa n dP l a n t s " ) . P. Nick or the Hidden - a : t. : . , t . L r i c .i'n p l a n t c e l l s , . ' . . : : r r p l r r n tc y t o s k e l e t o n . . i . ' r . r t i o n sI.t w a s P a u l ..:,,',l\ fepartition growth ' 1.i..','Ll\l\ pref'emed lateral ''.'.cll c l n e s t a b l i s ha n d . : .r.-) ct-unknown lattice I i : : i n 1 9 6 2 )T . h u s ,e v e r ..: i r r k c t lu i t h m e c h a n i -: ,:-' n()ttirllow the usual , .. .,: irusisand then derive J , ,.l, '. kc leton that derives - . . : : ] l (\ t ' P h o - q e n e s i s . . . : . : . r n a r e a s et h e i r s u r f a c e - . i r r r n i c al o a d . A s l o n g . : - , b L r o v a n c ya, l l o w i n g : - . I lr rri cVer, when plants j.-. ,i, flerible yet robust : . . . r ' r r l u l c s t. h e s o - c a l l e d ' 1 .- .'\ olutionary Success - , . L r i : ul e v e l . P l a n t c e l l s . ' . ' n ; i n t lc e l l e x p a n s i o n '.I - : , , n r c c h a n i c so f C e l l ' . ..llirll\ of mechanical :. :- iontplex interplay It i: even possible - ., \ !t rrranit'estationof - r .' :tt.oltginfluence of . . . ' , , r r rbl r c h a n g i n gt h e - . - . r r n r lf i b r e s o n t h e . : thc cellular level) [: . '': .' . i. the fbrmation : . r , . r . l .1r0 0 8 , 1T. h i s . ..:r..rl irr an optimal . - : . t h i sm e c h a n i c a l .r nlti\l ln a syslem : L r , L l ler x t e n d e db y I , , : . e R e s p o n s e so f Mechrnics ol lhe C) tO\keleton 5-5 It was only'in the 1920swhen such self-supporting, flexible structureswere deliberatelyconstructedby mankind (Robby 1996).Startingfrom the Equilibrist Studies (consistingof three solid sticks interconnectedby ropes) of the Soviet artist Karl Joganson,it was mainly the American architect and engineerRichard BuckminsterFuller (1895-1983)who systematically adoptedbiologicalprinciples and developedself-supportivestructuresthat all consistedof a continuousnetwork of tensileelements(which can transmitforcesby pulling) linked to a discontinuoussystemof stiff elements(which can transmitforcesby pushing).lt was also Buckminster Fulier who later coined the term "tensegrity" as a combination of "tension"and "integrity". A few yearslater it becameclear that animal cells are shapedby tensegrit)'(for reviews see Ingber 2003a, b; chapter"Introduction: Tense-eralWorld of Plants"). The part of the tensile elementsis played by actin microfilaments,which are not only contractile,but are also mechanicallycomparableto silk fibres (Gittes et al. 1993).The part of the stitT elementsis played by the microtubules,which are not only hollow cylinders, but are also mechanicallymuch more rigid than actin filamentsand can be approximated as very delicateglasslibres(Gitteset al. 1993). The actinfilamentsareconnectedthroughthe membranewith a suppofiingscaffbld the extracellularmatrix. What is the secretthat rendersbiological tensegrityso successful?Biological tensegrityis not constructeda priori, but emergesa posteriorifrom reorganization in responseto ever-changing patterns.To usea term ofJacob (1977). stress-strain biological shapeis not producedby intelligentdesignbut ratherfrom bricolctge,and therefbreit oscillatesaround the stateminimal energy without every reachingil. Plantcellsareendowedwith a cell wall that is built asa compositestructurewith elongateload-absorbingelements(cellulosemicrofibers)that are embeddedin an amorphousmatrix (hemicelluloses, pectins,proteins).It hasbeenshownfor technical applicationsthat suchcompositematerialsoptimallycombinebendingflexibility with mechanicalstabiiity(Niklas 1992).This meansthatthe tensegrityfunction fulfilled by the interphase cytoskeletonin animalcells is replacedby tensegrityof the cell wail. The plant cytoskeletonis thereforenot directly required to support cellular architectureand is thereforefree to adopt other functions (Fig. I ). Cellular architectureusesthe tensegralprinciple to achievemaximal mechanical stability and, simultaneously,flexibility on the basis of parsimonioususe of resourcesand load-bearingelements.In addition,it adaptscontinuouslyto the ever-changing conditionsof growing and developingcells.This requiresefficient sensingofforces and strainsfbllowed by appropriatereorganizationofthe tensegral elements.Thus,the tensegralcytoskeleton is not only a deviceto providemechanical stability.it mustalsoparticipatein the sensingof stressand strainpatterns.This mechanicalstimulationf'eedsback to the organizationof the cytoskeletonin sucha way that a stableminimum of mechanicalenergy is reachedand continuously adjusted.It is this hidden face of tensegritythat becomesespecially important in the walled plant cells that are undercontinuousturgor pressureand usethis pressure fbr regulatedexpansion.The evolutionof the interphasicplant cytoskeletonwas thereforeshapedby selectivepressurestowardsoptimized sensingand integration P. Nick 56 Animal cell - architectural tensegrity b Plant cell - dual tensegrity Misotubules:compression sensory tensegrity cytoskeleton: cell wall: architecturaltensegrity Fig. 1 Functional shift of the cytoskeleton from architectural tensegrity in animal cells towards sensory tensegrity in plant cells. In animal cells (a), architectureresults from the interplay between stiff microtubules absorbing compressive forces and flexible actin filaments elastically tethering the cells through focal adhesionsto the substrate.This set-up is used both to maintain cell shapeand to senseand to adjust to mechanical force. In plant cells (b), cellulosic microfibers in combination with a flexible cell wall matrix maintain cell shape,such that the tensegralcytoskeleton is released from its architectural function and can be optimized for sensory integration ofmechanical stimuli of mechanicalstimuli. The aim of this review is to give a survey of the role of the cytoskeletonin mechanicalintegrationof plant cells, tissuesand organs. 2 Cellular Players:The Microtubule-Actin TensegritySystem The plant cytoskeletonconsistsof two major elements,microtubules and actin filaments - intermediatefilaments have remained elusive in plants. The spatial organization of these elements is highly dynamic and changes fundamentally P. Nick tectural tensegrity Mechanics of the Cytoskeleton 51 during the cell cycle, with conspicuouseffects on the predicted stress-strain patterns.Since microtubules and actin filaments are functionally, but probably also structurally,interconnected(Wasteneysand Galway 2003; Collings 2008), it seemsmore appropriateto describe their dynamics as a functional entity. The molecular players have been extensivelyreviewed elsewhere(for recent reviews seeHamada2007; Sedbrookand Kaloriti 2008),and thus the focus of this chapreris on cellular function, ratherthan on molecular composition. 2.1 Cell Expansion 2.1.1 Microtubules rll - dual tensegrity . ' c h , t e c t ur a l t e n s e g r i t y '- -rrrr in animal cells towards u t- .. : . , i l ! ' n t \ e l a s t i c a l l yt e t h e r i n g . ,.. I t : . t ( Jn t a i n t a i nc e l l s h a p ea n d i - . . . ; r r er t r f i b e r si n c o m b i n a t i o n -. . . : : . i l i r t o s k e l e t o ni s r e l e a s e d - -:.rrrt)lo t l m e c h a n i c a ls t i m u l i :. . i .: .ur\ e1 of the role of the - : , . . . - , \u n do r g a n S . -Actin TensegritySystem '-..:: -':.:. ::l!rotubules and actin s d - - - . . , ' r n p l a n t s .T h e s p a t i a l trrr- .:. : ,h.rnges fundamentally In interphasecells, microtubulesare organizedin arraysofparallel bundlesperpendicular to the axis of preferentialcell expansion(Fig. 2a). Thesebundlesarethought to control the direction of cellulosedepositionand thus to reinforce the axiality of cell growth. Corlical microtubules can change their orientation in responseto variousexternaland internalstimuli, and this reorientationwill shift the preferential direction of cellulosedepositionand thus the mechanicalanisotropyof the yielding cell wall suchthat the proportionalityofcell expansioncan be alteredin responseto the stimulus(for a recentreview seeNick 2008a).The cell wall in cells that are not endowedwith tip growth is formed by appositionof celluloseon the inner surfaceof the cell wall. Cellulose is synthesizedby specializedenzyme complexesthat, in freeze-fracturepreparations,appearas rosettesof six subunitsof about 25-30-nm diameterarounda centralpore (Kimura et al. 1999)and are thereforedesignatedas terminal rosettes.The terminal rosettesare integratedinto the membraneby fusion of exocytotic vesicles.UDP-glucoseis transportedtowards the central pore and polymerizedin a B-1,4 configuration.Each subunit of the cellulose synthasewill producesix cellulosechainsthat will be integratedby hydrogenbonds into a long and fairly stiff cellulosemicrofibril. Theseenzymecomplexesare thoughtto move within the fluid membraneand leave a "trace" of crystallizing cellulose behind them. This movementwill thereforedeterminethe orientationof cellulosemicrofibrils and thus the anisotropyof the cell wall. It is at this point that the microtubules come into play. ln fact, it was cell-wall anisotropythat led Green (1962) to predict that microtubulesmust exist even beforethey were actuallydiscoveredmicroscopically by Ledbetterand Porter (1963). The intimate link between cortical microtubules and the preferential axis of growth is supportedby the following main arguments(for a review seeNick 2008a) (l) upon plasmolysis, direct contact between cortical microtubules and newly formed cellulose microfibrils can be demonstratedby electron microscopy; (2) when the axis of cell expansion changes in responseto a stimulus or during development,this is accompaniedby a correspondingswitch in the preferential axis of cellulosedeposition,precededby a correspondingreorientationof cortical microtubules; (3) elimination of cortical microtubules by inhibitors produces a 58 a P. Nick c F i g . 2 M o l e c u l a r p l a y e r s o f m e c h a n o i n t e g r a t i o ni n e x p a n d i n g ( a . c ) a n d d i v i d i n g ( b . d ) p l a n t cells. Microtubules and actin filaments cooperate to fbrm tensegral structures together with other proteins. (a) Organization of microtubules (M7s) and actin filaments in an expanding cell; ntolecular details o1'the cell wall-plasma menrbrane*cytoskeleton(WMC) continuum are shou'n in (c). (b) Organization ofthe microtubular preprophaseband (PP^B),and the actin-based phrag, mosome in a cell preparing for mitosis; molecular details of the nuclear envelope are shown in (d). (c) Molecular details of the cell wall-plasma membrane{ytoskeleton continuum. PLD phosphoIipase D anchor for microiubules. CE,Scellulose synthasecomplexes that are pulled by kinesins of the KIFzI family along microtubules towards the plus-end complexes (+I1P) that are linked via c r o s s - w i r i n g p r o t e i n s ( C r W i P s ) s u c h a s k i n e s i n s c o n t a i n i n g a c a l p o n i n - h o m o l o g yd o m a i n w i t h actin, putative plant homologues of integrins (lntH) and mechanosensitive ion channels (MSC). The functional integrin homologues are linked with the exiracellular matrix (ECM). e.g. arabinogalactan proteins, and cellulose n-ricrofibrils.(d) Molecular details ofthe link berween the nuclear envelope and the cytoskeleton. Through cross-wiring proteius, a tensegral link between aetin lilaments and microtubules is generated, whereby microtubules might confer compression forces between the periphery and the nucleus. whereas the flexible actin filaments transfer tension fbrces. NPC nuclear pore complex progressiveloss of orderedcellulosetexture and the axiality of cell expansion, leading, in extreme cases,to lateral swelling and bulbous growth. The mode of action of severalherbicide classes,such as the phenyl carbamatesor the dinitroanilines.is basedon the eliminationof cortical microtubulesand the subsequent inhibitionof elongationgrowth. The striking parallelismbetweencofiical microtubulesand newly deposited cellulosemicrofibrilsstimulatedtwo alternativemodels:The original "monorail" model postulatedthat cofiical microtubulesadjacentto the plasmamembraneguide the movementof the cellulose-synthesizing enzymecomplexesandthusgeneratea patternof microflbrilsthat parallelsthe orientationof microtubules(Heath 1974). The driving force for the movementof cellulosesynthases in the monorailmodel P. Nick --) -4 act:' V- *s 7 - i 'y'renston ' t-:r lj3'. r c and dividing (b. d) plant . i l u . t u r e s t o g e t h e rw i t h o t h e r .i:r.'nt\ in an expanding cell; ri l/C) continuumare showrr .', . untl the actin-basedphrag. . u c n v e l o p ea r e s h o w n i n ( d ) . ' ,n e o n t i n u u m .P I D p h o s p h o - - : h , r ta r c p u l l e d b y k i n e s i n so f ' . T l P t t h r t a r e l i n k e dv i a : ' , : r r n - h o n t o l o g yd o m a i n w i t h - . ' r \ 1 l i \ e i o n c h a n n e l s( M S C ' ) . :rirtri\ (ECM), e.g.arabinot.' . r : . ,, r n k b e t w e e nt h e n u c l e a r . : r : r l l i n k b c l u e e na c l r n - , L r n t e rc o m p r e s s i o nl b r c e s - r r \ l r a n \ f e r t e n s i o nf o r c e s . ,litr of cell expansion. { r ( ) \ \ t h .T h e m o d e o f i..rnr.rtes or the dinitror. .rnclthe subsequent rnrl newly deposited , r r i g r n a l" m o n o r a i l " .ir;.i rnembraneguide ' unil thus generatea r i . L r l e (sH e a t h 1 9 7 4 ) . tir.' nronorail model Mechanics of the Cytoskeleton -59 would be an activetransportthroughmicrotubulemotors.Alternatively.the interaction between microtubulesand cellulose synthasescould be more indirect. whereby the microtubulesact as "guardrails" inducing small fblds of the plasma membranethat confine the movement of the enzyme complexes (Giddings and Staehelin199I ). The driving force for the movementwouid be the crystallizationof cellulose.The solidifyingmicrofibrilwould thuspushthe enzymecomplexthrough the fluid plasma membrane,and the role of microtubuleswould be limited to delineatingthe directionof this movement. The practical discriminationbetweenthesetwo models is far tiom straightforward becauseexperimentalevidencewas mostly basedon electronmicroscopy observationand thus prone to fixation artefacts.Moreover, great luck was required cellulosemicrofibrils to locatethe right section.For instance.the newly synthesized fbrmed afier treatmentwith taxol were sometimesfound to be directly adjacentto individualmicrotubules. fbr instancein tobaccoBY-2 cells(Hasezawaand Nozaki 1999),supportingthe monorail model. On the other hand,the cellulosesynthase complexeswere observed"in gap" betweenadjacentmicrotubulesin the alga Closterium(Giddingsand Staehelin1988),favouring the guardrailmodel. The situation was further complicatedby situationswhere the orientationsof microtubulesand cellulosemicrofibrilsdiff'er(for a review seeBaskin2001;Wasteneys 2004),leadingto a debateon therole of microtubulesin guidingcellulosesynthesis. This debate stimulateda key experimentexploiting the potential of live-cell imaging in Arabidopsisthaliana (Paredezet al. 2006).A componentof the terminal rosette,cellulosesynthasesubunit A6 (CESA6), was expressedas fusion with yellow fluorescentprotein under the native promoter in the background of a cesa6-null mutant, such that overexpressionaftefacts could be excluded. The resultingpunctuatesignal was observedto be localizedadjacentto the plasma membraneand to move along parallel pathwaysthat resembledcortical microtubules.By crossingthis line into a background.v'hereone of the s-tubulinswas protein,it becamepossibleto fbllow this expressed as lusionwith a blue fluorescent movementundersimultaneous visualizationof CESA6andmicrotubuies. This dual visualizationdemonstrated very clearlythat CESA6 was moving along individual microtubule bundles.Moreover. in a recent publication. a central problem of the monorail model, i.e. the existenceof polylamellate walls with layers of differing microfibril orientation, could be plausibly explained by a rotary movement of groups of microtubules(Chan et al. 2007; for a recent review see Lucas and Shaw2008). The original monorail model postulateda microtubule motor that pulls the cellulose svnthasecomplex along the microtubules.lf this motor were defective. cellulosemicrofibrilswould deviatefiom the orientationof microtubules. A screen for reducedmechanicalresistance in A. thalianayieldeda seriesof so-calledliagrle fiber mutants(Burk et aI.2001: Burk and Ye 2002) that were shown to be completelynormal in tems of cell wall thicknessor cell wall composition,but were afl-ectedin terms of wall texture.One of thesemutants,.fi'agilefiber 2, allelic to the mutanthotero(Bichetet al. 2001).was affectedin the microtubule-severing proteinkatanin.leadingto swollencellsand increasedlateralexpansion.A second 60 P. Nick mütant,fragilefiber -1,was mutatedinto a kinesin-relatedprotein belongingto the KlF4 family of microtubule motors (Ftg. 2c). As expected,the array of cortical microtubules was completely normal; however, the helicoidal arrangementof cellulose microfibrils was messed up in these mutants. This suggeststhat this KlF4 motor is involved in guiding cellulose synthesisand might be a component of the monorail complex. Thus, the original monorail model for microtubule guidanceof the terminal rosettes(Heath 1974) was rehabilitatedafter more than three decadesof dispute. However, the microtubule-microfibril model is still far from complete. ln addition to occasionally discordant orientations of microtubules, there are cell wall textures that are difficult to reconcile with a simple monorail model. For instance. cellulose microfibrils are often observed to be intertwined (Preston 1988).This has stimulatedviews that claim that microtubulesare dispensablefor the correct texture of microfibrils. The self-organizationof cellulose synthesis would be sufficient to perpetuatethe pattern becausethe geometricalconstraints from microfibrils that are alreadylaid down would act as templatesfor the synthesis of new microfibrils (for a review seeMulder et aL.2004').There are two problems with this model. First, it ignores that microtubules and microfibrils are actually parallel in most cases,at least if cells in a tissuecontext are analysed.Second,it ignoresthat disruption of microtubuleseither by inhibitors (for a review seeNick 2008a,b) or by mutationsthat impair the formation of orderedmicrotubule arrays (Burk et al. 2001; Bichet et al. 2001 for katanin;Whittington et al. 2001 for mor l) \s accompaniedby a progressiveloss of orderedcell wall texture and a loss of growth axiality. Nevertheless,the issue of cellulose self-organizationhighlights that the original microtubule-microfibril model hasto be extendedby a feedbackcontrol of microfibrils upon cortical microtubules. 2.1.2 Actin Filaments Similar to microtubules,actin is organizedinto severaldistinct arraysthat presumably exert different functions.For cells with pronouncedtip growth, suchas pollen tubesor root hairs, actin functionsas a track for the transpofi of vesicleswith cellwall material that are insertedinto the tip by intussusception(reviewed in Hepler Mechanicsof Tip Growth") et al. 2001;chapter"Generatinga CellularProtuberance. and are probably conveyedby actin polymerization (Gossotand Geitmann200l; Cärdenaset al. 2008). However, cells growing in a tissuecontext grow by apposition to the stretchedcell wall rather than by intussusception.The role of actin therefore must be different and is not as obvious as for tip growth. During the diffuse elongationof tissue cells, longitudinal actin bundlesprevail, especiallyin vacuolated cells (Parthasarathyet al. 1985; Sonobe and Shibaoka 1989). The rigidity of thesetransvacuolarstrandsand the degreeof their bundling is regulated by signalssuch as plant hormones(Grabski and Schindler 1996),kinase cascades (Grabski et al. 1998) or light (Waller and Nick 1997'1.\naddition to the transvacuolar bundles,a fine network of highly dynamic microfilamentscan be detectedin P. Nick rn-:: .,iJli irrr)teinbelonging to the {. ;'.;.,'.ted. the array of cortical : . : : . J h . l l e ( ) i d a l a r r a n g e m e n to f r',-i-:i'.i. Thrs suggests that this r!h.... .,n!l nticht be a component :::.'r' ,:.rrLnrodel for microtubule ^r- :rit.rbilitated after more than :. .. .trll t'al fiom complete. In . . : :lt!r()lLlbules, there are cell lh r .i:lpic ntonorail model. For ':-.r-: r,, he intertwined (Preston I :r:. :,'rubules are dispensablefor jii-.:./.ilr()n of cellulose synthesis '.:-.- tlri rcometrical constraints rl ;. i .:. tlnrplates for the synthesis . . _ rrr_,. There are two problems u.i. .,irti tntcrofibrils are actually . -,,:'.1.'\tale analysed. Second, it :n:..ill\\r\ (lbr a review seeNick r..':'.': ,Jrlleredmicrotubule anays \ i:::.r!t()n et al. 2001 for morl) is ^ .r .:. . i r'\ t ure and a IOSSOf growth .:-,':i.rnrllrion highlights that the :r:::r.i.'.i br a f'eedbackcontrol of < r r : . : . . : t . t l n c l a r T a y st h a t p r e s U m t" r*: , r.- llI rrowth, such as pollen t:< ::-:: .p1r11 llf veSiCleSwith Cell- \ . - . , , . ; r i r ( ) l tl r e v i e w e d i n H e p l e r ::::.^-: \lrihanics of Tip Growth") !),.:.. r : ..rrt .rnd Geitmann 2007; ' - : i . . . . r . ( ) n t e x tg r o w b y a p p o s i ni-.....-:|tl()ll. The role Of actin r-. ":. r : trp growth. During the : . : : : - . . . . . : . J \ p r e v a i l , e s p e c i a l l yi n rf,r .-.: .,r .i Shibaoka 1989). The ::.:rr bundling is regulated F:.i : : - . : i 9 9 6 t . k i n a s ec a s c a d e s i' .+, : . r J . l i t i o nt o t h e t r a n s v a :1r- - ..::]int\ can be detected in Mechanics of the Cytoskeleton 6l the corlical cytoplasm of elongating cells often accompanyingcortical microtubules (for a review see Collings 2008). This corlical network can be rendered visible after pretreatmentwith protein cross-linkers(Sonobeand Shibaoka 1989), upon very mild fixation (Waller and Nick 1991)or by fusing binding domains of actin-bindingproteinsto fluorescentproteins(Voigt et al. 2005; Nick et al. 2009). The transvacuolaractin cableswere suggestedto limit cell expansionby their rigidity and auxin was thought to stimulate growth by releasing this rigidity (Grabski and Schindler 1996). This mechanical model for the growrh control through actin was supported by experimentswhere growth was modulated by light (Waller and Nick 1997).ln the dark, when cells underwentrapid elongation, actin was organized into fine strandsthat became bundled in responseto lightinduced inhibition of growth. This actin reorganizationwas rapid and preceded the changesin growth rate (Waller and Nick 1997).Moreover, this responsewas confined to the epidermis, the target tissue for the signal control of growth. However, the mechanicalmodel of actin function was shatteredby experiments with actin inhibitors. The mechanicalmodel predicted that elimination of actin cables should releasethe rigidity that limits growth. However, experimentswith cytochalasinD (Thimann et al. 1992:-Wang and Nick 1998) and latrunculin B (Baluika et al. 2001) revealedthat even mild elimination of actin inhibited. rather than promoted, cell elongation.Thus, actin, possibly in combination with directional vesicletransport(Baskin and Bivens 1995),is a positiveregulatorof growth. Subsequently,two actin populationscould be separatedowing to differencesin sedimentability(Waller et al. 2002). Whereasthe fine actin filaments correlated with a cytosolic fraction of actin, actin became progressively trapped on the endomembranesystemand partitionedinto the microsomalfraction when bundling was inducedby light (perceivedby phytochrome),by fluctuationsof auxin contenr or by brefeldin A. This bundling of actin was accompaniedby a reduced auxin sensitivityof cell elongation.This led to a model where auxin-signallingtriggered the reorganizationof actin bundlesinto finer filamentsthat more efficiently transported auxin-signalling/transport componentstowards the cell pole. The debundling in responseto auxin predictedby this model was later demonstratedin intact rice coleoptilesin vivo usingthe actin-bindingdomainof mousetalin in fusion with yellow fluorescentprotein first upon transientexpression(Holweg et al. 2004) and later after stableexpression(Nick et aI.2009). To understandthe link betweenactin and the auxin responseof growth, excessive actin bundling was inducedby overexpressionof the actin-bindingdomain of talin in tobaccoBY-2 cells (Maisch and Nick 2007) and in rice plants (Nick et al. 2009). In both systems,the reversion of a normal actin configuration can be restored by addition of exogenousauxin and this fully restoresthe respective auxin-dependentfunctions.These findings led to a model of a self-referringregulatory circuit betweenpolar auxin transportand actin organization.Thus, although actin can stimulate growth by virtue of its mechanicalpropertiesin tip-growing cells (Gossotand Geitmann2007), within a tissuecontext it does not act through mechanics,but acts by controlling the proper localization and thus activity of the signallingmachinerythat regulatescell expansion. 62 P. Nick 2.1.3 Actin-Microtubule Interaction For cell growth, coordinationand cross talk betweenmicrotubulesand actin fila(for mentshavebeeninf'erredliom their closecoalignmentand structuralinteraction ubiquitous to be seem that reviewsseeWasleneysandGalway2003;Collings2008) andhavebeenobservedin differentspeciesandcell types.This conclusionhasbeen manipulation,where both supporledby experimentsinvolving pharmacological cell elongation'otten reduced agents microhlament-and microtubule-eliminating andBivens 1995: Baskin (Arabidopsis: by a lossof growthanisotropy accompanied et al. 2004: Wang 1998; et al. Gianf collings et al. 2006; Gramineanseecllings: proposed been even it has Blancaflor2000;cottonfibres:Seagull1990).Recently, memfrom microtubules of that microtubulereorientationis causedby cletachment D: phospholipase of contactpoints(controlledby specificisoforms brane-associated streaming of actin-based Fig. 2c) followeclby their realignmentwith the direction (Sainsburyet al. 2008).Despiteextensivestudieson microtubularassociationof actin filaments,the proteinsthat mediatetheseinteractionshave remainedelusive. Interactionsbetweenmicrotubulesand actin filamentscould be mediatedeither by bifunctionalproteinsthat can bind to both cytoskeletalelementsor, alternatively.by a connectingcomplex of two or more monofunctionalproteinsharbouringa microIn animalandfungalcells. or an actin-bindingdomain,respectively. tubule-binding a numberof proteinshave beenidentiliedthat mediatesuchinteractions.either in a (Goode bifunctional way or as complexesconsistingof monofunctionalproteins suchas candidates a few et al. 2000;Rodriguezet al. 2003).ln plants,however.only MAPl90 havebeenproposedso fär (Igarashiet al. 2000)' In animal cells. the microtubularminus-endmotor dynein is connectedwith and activateclthrough the clynactincomplex (for a review see Karki and Holzbaur 1999).The dynactin complex is further linked to the microtubule tip component EBI and thusregulatesthe stabilityof microtubules(fbr a review seeTirnauerand Bierer 2000).Dyneins as centralelementsof the dynactincomplex are not fbund in of the lossof flageliatecellsandcentrioles plants.This is probablythe consequence (for a review see Schmit and Nick 2008). Plantsmust have evolveda functional compensationfor the loss of dyneins in the fbrm of other minus-end-directed motors that are able to interact,either directly or indirectly. with actin filaments. domain(KCH kinesins)were identiRecently,kinesinswith a calponin-homology lied as plant-specificsubsetof the kinesin-14fämily (Tamuraet al. 1999;Preuss et al. 2004; Frey et al. 2009; Xu et al. 2009). In addition to the characteristic microtubule-bindingkinesin motor domain, these proteinspossessa conserved domain.well known as an actin-bindingdomainfiom a variety calponin-homology proteinssuchas q.-actinin,spectrinand fimbrin. Thus. KCHs are of actin-associated for a bifunctional mediationbetweenboth cytoskeletalelements. strongcandiclates and several studies confirmed that they can bind, in fact, both elementsof the cytoskeleton(cotton:Preusset al. 2004: Xu et al. 2009; rice: Frey et al. 2009). These cross-linkingmicrotubule motors are presentin higher plants, but also in Pltvslornitrella ytatens(Richardsonet al. 2006; Frey et al. 2009)' Their cellular function is not really understood,but for one of the cotton KCHs, a role in cell T_ P. Nick ,\ --'r , : ( ) t L l b u l e \a n d a c t i n f i l a :..: .. .: .ll.ueturalinteraction (for - - . . irt,it \eerr to be ubiquitous r . : . . ' - ' . l h i . c o n c l u s i o nh a s b e e n , i . - . . . r : r . r n i p u l a t i o nw, h e r e b o t h r - - r . . - i ' i i . c l l e l o n g a t i o n .o f i e n . -it r:: - Il.r:kinand Bivens 1995: lei)S: Wang et al. 2004: ir h.t\ even been proposed , nrrenrtubulesfiom memr : - .,:,)rrll\ofphospholipaseD: ! . : - ' - . , r , , 1u c t i n - b a s e ds t r e a m i n g : - " : . : - : .' n . h i r r e r e m a i n e de l u s i v e . r - - :: . - , L r , ,bi c ' m e d i a t e de i t h e r b y l:... - . r:)ent\ ol'. alternatively, by - . : i ' 1 . ' r r lh: r r r b o u r i nag m i c r ( ) : . . - . . l r r i n i n r a la n d f u n g a l c e l l s . . i . . : r - . . r . h i n t e r a c t i o n se, i t h e r i n a ::. ' . J' : . . n ! t i ( ) l r a lp r o t e i n s ( G o o d e : .. \ .r lL-\\candidatesSuchas -, . , , : . : r n i : c o n n e c t e dw i t h a n d , :' , : . ( ) t t l b L l ltei p c o m p o n e n t .'. .. ::r ieu see Tirnauer and :.- . - ' J i n p l e xa r e n o t f o u n d i n ' .. - - ' . , . r 1 c! 'e l l s a n d c e n t r i o l e s . ": ... ....' i\olved a functional :-:irlr nrinus-end-directed r . - -r.,.. *ith actin ltlaments. :: r .'\i . t- - r r l 1 r . i n e s i n sw ) ere identi. . r . r l i .cr t a l . 1 9 9 9 ; P r e u s s ':, I,\ the characterislic - . Ir()\\eSSa COnSefVed - t,'rrrrinfromavariety 'r::l.r'irT r .h u s .K C H s a r e ' . r 1 ',. l s l s 1 1el l e m e n l s . L r :: - : i . , , t he l e m e n t so f t h e ' -. Fler el aI.2009). . u t a l s oi n -irrr plj115b 1lr 191.Their cellular ' K ( l l \ . a r r l l ei n c e l l t Mechanics of the Cytoskeleton 63 elongation through cross-linking of microtubules and microfilaments has been proposed,howeverwithout experimentalevidence(Xu et al. 2009). For the rice homologueOsKCHl, the phenotypeof insertionmutantsand a KCH overexpressionline generated in tobaccoBY-2 cells(Freyet aI.2010)suggestthatthis kinesin phenomstimulatescell elongation,althoughthis stimulationmight be a secondary enoncausedby changesin the timing of cell division. 2.1.4 The Cell-Wall CvtoskeletalContinuum A continuum betweenthe cytoskeletonand the extracellularmatrix is central fbr mechanosensing in animalcells(1bra review seeGeigerand Bershadsky 2001)and involves interactionbetweenintegrinsand extracellularmatrix proteinsthat contain Arg-Gly-Asp (RGD) motifs (for a review seeGiancottiand Ruoslahti 1999).Plants seemto lack integrin homologues,but there is evidencefor cytoskeletalreorganizationin responseto treatmentwith RGD peptides(Canutet al. 1998;Wang et al. 2007). As a molecular basis for the cytoskeleton-plasmamembrane-cell wall procontinuumin plants (Fig. 2c), cell-wall-associated kinases.arabinogalactan (for a revrew teins,pectinsand cellulosesynthases themselves havebeendiscussed see Baluika et al. 2003; chapter "lntroduction: TensegralWorld of Plants"). Recently,the rich but circumstantial evidencefor suchtransmembrane interactions was assembledinto a model for a so-calledplasmalemmalreticulum as a third elementof the plant cytoskeleton(Pickard2008).This plasmalemmalreticulumis consideredto be a tensile structureand to participatein the control of cellulose deposition.It can be visualizedas a reticulatestructureby antibodiesraisedagainst componentsof the mammalian extracellularmatrix and also contains arabinogalactanproteins.The reticulumis reorganizedin the contextof cell elongationin a mannersimilarto cellulosedepositionand is suqgested to representa morphological manifestationof lipid rafts. 2.2 Cell Division 2.2.1 Microtubules ln dividingcells,the ensuingmitosisis heraldedby a displacement of the nucleusto thecell centre,wheretheprospective cell-platewill be fbrmed(fbr a reviewseeNick 2008a).Simultaneously,radial microtubulesemanatefiom the nuclearsudaceand merge with the cortical cytoskeleton,tetheringthe nucleusto its new position (Fig. 2b). In the next step,the preprophaseband is organizedby the nucleusas a broad band of microtubulesaround the cell equator,marking the site where after completedmitosisthe new cell plate will be formed.In fern protonemata.wherethe formation of the preprophaseband can be manipulateclby centrifugationof the nucleusto a new location (Murata and Wada 1991).a causalrelationshipbetween P. Nick the preprophaseband and cell-plateorientationwas elegantlydemonstrated'Moreover, in cells where the axis or symmetry of cell division changes,this changeis always predicted by a correspondinglocalization of the preprophaseband. The division spindle is always laid down perpendicularto the preprophaseband, with the spindle equatorlocated in the plane of the preprophaseband' As soon as the chromosomeshave separated,a new array of microtubules,the phragmoplast' appears at the site that had already been marked by the preprophaseband' This microtubularstructureconsistsof a double ring of interdigitatingmicrotubulesthat increasesin diameter with increasingsize of the cell plate. New microtubulesare organizedalong the edge of the growing phragmoplast(Vantard et al. 1990).The phragmoplasttargetsvesicle transportto the peripheryof the expandingcell plate. Microtubules seem to pull at tubular-vesicularprotrusionsemanating from the endoplasmicreticulum(Samuelset al. 1995).The guidingfunctionof the preprophase band is supportedby evidence from Arabidop.sismutants (tonneaulJass),where the preprophaseband is absentowing to a mutation in a phosphatasePP2A regulatory subunit(Camilleri et a|.2002).In thesemutants,the orderedpatternof cell divisions that characterizesthe development of the wild type is replaced by a completely randomizedpattem of crosswalls (Traaset al. 1995;McClinton and Sung 1991)'It should be mentioned,however,that during meiosisthe division plane can be controlled in the absenceof a preprophaseband (for a recent review see Brown and Lemmon 2007),suggestingthat thereexist additionalmechanismsof spatialcontrol. 2.2.2 Actin Filaments In contrastto the obvious and dramatic reorganizationof the microtubule during mitosis, actin filaments seem to be more persistent.Two decadesago, actin filamenls were shownto accompanymitotic mic:otubulearrayssuchas preprophase band, spindle and phragmoplast(Kakimoto and Shibaoka 1987; Lloyd and Traas 1988).However, there is still some controversyas to the exact behaviour,persistence and orientation of actin filamentsduring M phase (for a recent review see Panteris 2008). The microtubular preprophaseband that disappearswith the breakdown of the nuclear envelopeleavesa so-calledactin-depletedzone as a negative imprint that later, upon reestablishmentof the daughter nuclei, will be the site where the new cell plate is formed. Despite some debateregardingto what extent this zone is completely void of actin or whether it just contains fewer actin filaments,there is a clear correlationbetweenthe actin-depletedzone and the site of the prospectivecell plate.When the actin filamentslining this depletionzone are eliminatedby inhibitor treatment,this will affect the subsequentcell division when the treatmentoccurs during the presenceof the microtubular preprophaseband' However. actin inhibitors will have only a marginal effect once the preprophase band has disappeared(Sanoet al. 2005). What is the actin-dependentfunction that definesthe cell plate? It might be linked to a belt composedof endosomesthat is laid down adjacentto the preprophaseband by joint action of microtubule-driven and actin-driven transport (Dhonukshe et al. 2005). This belt persists during P. Nick r :. :.- :.!nti) demonstrated. More^ - J . . . r { } nc h a n c e st,h i s c h a n g ei s \\.. : ihr preprophaseband. The r..: : ' rhj preprophase band,with p:.t-:,,rh.r\eband.As soon as the : : . - : , , t L r b u l etsh. e p h r a g m o p l a s t , i.t :. ihr preprophase band. This .: :::l::Jrgrtatingmicrotubulesthat : -.. l.irtc. \ew microtubulesare n , ' r . . : . i V a n t a r de t a l . 1 9 9 0 ) T . he rrn.:\ \)1the expandingcell plate. ' l:,,l:u\l()ns emanatingfrom the J!r.r:irLIunctionof thepreprophase :r -:.1:rl\\ t ()tlleauffass),where the ,.. ., ;.ilir:phatasePP2A regulatory :ht ,,:.irrerlpattemof cell divisions :\t\i r. rcplacedby a completely r.r: \1.('linton and Sung 1997).It hr. ::'..'Jir isionplanecan be conr. r :J.Jltt review seeBrown and -{:r- ::Jjhanisms of spatialcontrol. r:1i: ,ir ,,1 the microtubule during !...: i.',,, clecadesago, actin fila(Ll,..: ,rr-rJ\s SuChaS preprophase \:..^.,,'x.rl9lt7: Lloyd and Traas : , I : : . r ' r ' \ u L Ib e h a v i O u rp. e r s i s \ t , . - . 1 ( ) ra r e c e n t r e v i e w s e e r:L l:..r: .ir.rtppsalt with the break:-: : - 1 r 1 . ' 1 .Z' io n e a s a n e g a l i v e . : r - : : ' : r : n L r c l e i .w i l l b e t h e s i t e F -:- ^..:-' :e-larding to what extent -,i. ...1 r'onlaifls fewer actin E j-: : --lrl)lcteclzone and the site rr::. : :nL rhis depletion zone are !.:.: .---','quent cell division when !: - - : . . h u l . r rp r e p r o p h a s eb a n d . E:i-; - r:--. i r)ltce the preprophase t ::^:i . -: : -Jtpendent function that :-1 - : :\ ,.:.1 Lrf endosomes that is s : : : . - - r . , n L r ln t i c r o t u b u l e - d r i v e n . nr' belt persists during --\r < Mechanics of the Cvtoskeleton mitosis,and is, upon completedseparationof the chromosome,"read out" by a new set of microtubulesemergingfrom the spindlepolesthat "explore" the cell periphery in different directions. The lifetime of these exploratory microtubules is increasedwhen they hit the endosomalbelt, whereas microtubules that fail to interact with the endosomesare prone to undergocatastrophicdecay (Dhonukshe et al. 2005). Thus, the actin-depletedzone is rather a manifestationof and not the causefor the correct positioningof the cell plate. 2.2.3 NuclearMigration In cells that preparefor mitosis, the nucleusis tetheredby the so-calledphragmosome forming the characteristic"Maltese cross" to its new position in the cell centre, and persists during the subsequentmitosis (Fig. 2b). The cytoskeletal reorganizations accompanyingmitosis assigna centralrole to nuclearmigration in the control of division symmetry,and to the preprophaseband for the control of division axis. Nuclear migration can be blocked by actin inhibitors such as cytochalasinB (Katsutaand Shibaoka 1988). However, microtubulesalso seem to be involved in nuclearpositioning,since antimicrotubularcompoundssuch as colchicine (Thomaset al. 1977) and pronamide(Katsutaand Shibaoka 1988)have been found to loosen the nucleus such that it can be displacedby mild centrifugation. This indicates again that proteins mediating actin-microtubular interaction are relevant for nuclear positioning. In fact, the plant-specific KCHs (Frey et al. 2009) were found to be dynamically repartitionedduring the cell cycle: in premitotic cells, KCHI was clearly aligned in a punctuatepattern along filamentous, mesh-like structureson both sides of the nucleus and on perinuclear filaments spanningover and surroundingthe nucleus.At the onsetof mitosis,KCH I retracted to both sides of the nucleus, but not in preprophasebands nor in the spindle apparatusnor at the division plate. During late telophaseand the beginning of cytokinesis,KCHI was shifted towards the newly forming nuclei and lined the filamentsthat tetheredthesenuclei to the peripheryand the new cell wall (Frey et al. 2010). Tosl7 ftcl21knockout in rice showedincreasedcell numbersin the seedling shoot, whereasoverexpressionof KCHI in tobacco BY-2 cells reducedthe cell number. This effect was assignedto a delay in the premitotic nuclear migration towardsthe cell centre,suggestingthat KCH regulatesnuclearpositioningand thus the progressionof mitosis (Fig. 2d). 3 MolecularPlayers:Protein ConformationVersus Stretch-ActivatedChannels Mechanicalintegrationrequiresthe perceptionof stress-strainpatterns.In turgescent plant cells, the expandingprotoplastexertsconsiderableturgor pressureupon the yielding cell wall. This cell-autonomouscomponent of mechanical load is 6t) P. Nick complementedby mechanicaltensionacrossthe tissuethat is causedby the limiting extensibility of the epidermis(for a recent review seeKutschera2008). Thus, any has to cope with this strong, but tonic mechanismfor plant mechanosensing backgroundstimulus. Essentially,there are two basic models for the molecularbasis of mechanoperceptlon: l. Stretchingof proteinswill changetheir conformationand createnew binding sitesfbr the recruitmento1 associatedproteins(for reviews see Janmeyand weirz 2004: Orr et al. 2006). channelsare ableto directly detectand respondto forcesfrom 2. Mechanosensitive the lipid bilayer. Such channelswill open when the plasma membraneis deformedor whenthe channelis pulledby a tether(for a reviewseeKung 2005). Mechanosensingby stretch-inducedconfbrmationalchangesis well supported for the adhesionof mammalian cells (fbr a review see Geiger and Bershadsky 2001). Here. the growth of focal contacts"where the actin cytoskeletonis tethered to the extracellularmatrix througha complex of associatedproteinsand integrins,is promotedby local mechanicalforce (Rivelineet al. 2001).A similarmechanosensing network was proposedfor plant cells, whereanaloguesof integrinslink the cytoskeletonat the inner face with the cell wall at the outer face of the plasma membrane(Jaff'eet a|.20021.However"the transferof this model from mammalian cells to plants is not straightforwardbecausethe molecular componentsdiffer considerably(fbr a review seeBaluika et al. 2003).lt is the cell wall with a completely difTerentset of moleculesthat replacesthe extracellularmatrix of mammalian cells. Furthermore,canonicalintegrins are obviously absentfrom plants. suggesting that the link betweenactin filamentsand the extracellularbindingsites must usedifferentgroupsof molecules.Further.althoughplantsand animalsshare severalactin-bindingproteins,importantcomponentsof focal conlacts,such as talin. do not exist in plants. On the other hand, there seem to exist integrin analoguesthal can bind to RGD tripeptidesin a way similar to the way integrins do in animalcells.It seemsthat classVIII myosinsand fbmins mi-ehtact as linkers betweenthe actin cytoskeletonand the plant analoguesof the extracellularmatrix. proteins(Baluika and kinasesand arabinogalactan such as cell-wall-associated that move along microtubules Hlavaöka2005). Additionally.cellulosesynthases (Paredezet al. 2006)tetherthe cytoskeletonto the cell wall, and severalmicrotuproteins.fbr instancephospholipase D (Gardineret al. 2001)and bule-associated (Wang microtubulesand the act as linkers between corlical MAPIS et aI.2007), plasma membraneThus, although the molecular componentsdiffer considerably from their animal counterpart,a contiguouslink betweenthe cytoskeletonand the cell wall doesexist and is commonly ref'enedto as the cell wall plasmamembranecytoskeletoninterface(Telewski2006). channelsin plantswas originallydiscovered The existenceof mechanosensitive in specializedcells,wherea touch stimulusinducedan actionpotential(Shibaoka leavesof Mimosapudic'a(Toriyamaand Jaffe 1966)such as in the seismonastic 1912),or internodalcells of Charo (Kishimoto 1968).From electrophysiological P. Nick 'r. - . r r . r r r hr t1l t h e l i m i t i n g \..t.ihc'rrr 2008).Thus, any . . thr. \l|ong. but tonic , ' , L r i r i rb a s i s o f m e c h a n o - :r .Lnrlcreate new binding :.'\ i!'\\ s see Janmey ancl ' .irrrlrc\pond to forces from ':r. yrl;1;1n1 membraneis ; .i rcr ierv see Kung 2005). i.::: .: -lr,rnges is well supported , . : . , . i ' . ' C i e i g e ra n d B e r s h a d s k y ::i ..-:in irtoskeleton is tethered '. l I \ l \ ) r c i n a\ n d i n t e g r i n si.s ., ; .\ :imilar mechanosenr-' .: .. '-.Lrc:of integrins link the . .:i r , ,\uter filce of the plasma t--: rr. nr()(lelfrom mammalian t: J .' . LrI ur components differ : I . the ccll wall with a com: - ' , " . . .- . l L r l l rm a t r i x o f m a m m a u '- ,,r.lr absent from plants, :.: .: ' - ,'.,rr.acellular binding sites . - . I r l i l n t \a n d a n i m a l s s h a r e :.ii -r- ' l o r i l l c o n t a c t s ,S u c h a S :: . . , i . r . n lt o e x i s t i n t e g r i n l .rl lt) the waY integrins r. .:- . :':trnrmight act as linkers t . r r . i \ t r i . l c e l l u l am r alrix, r -. - .irr It'()tcilt\ (Baluika and 'ri alongmicrotubules {\' h.: - . .,.,. .rnti several microtu. " : , - , i , . , r ' t l i r l e t ' eatl . 2 0 0 l ) a n d .,-: r 'r' .. :.' . -.r lric|otubules and the -':rt' tlift'er considerably r : t J . \ t o s k e l e t o na n d t h e .. .t,1 plasma membrane- : ' si i r, +r . . . , , r ' i g i n a l l yd i s c o v e r e d - : , r r| ( ) t c n t i a l ( S h i b a o k a , I Lrr.irama and Jaffe : : :icitrophysiological M e c h a n i ts o f t h e C y t o . k e l e t o n 6l and phamacological evidence, a model emerged where these touch-sensitive channelsmediatean influx of calcium (for a review seeJaffeet al. 2002).In lact. with useof aequorin-transformed plants,mechanicalstimulationwas demonstrated to triggercalciuminflux with stimulus-specific signatures(Knight et al. 199l). A causativerole of calciumfluxeswas supportedby the isolationof touch-inscnsitive ArahidopsismutantsafTectedin calmodulingenes(Braam and Davis 1990),and inhibition of touch responsesby inhibitors of calmodulin (Jonesand Mitchell 1989). A mechanosensitive calcium channel was demonstratedfbr epidermal cellsof onion (Ding and Pickard1993).but the molecularidentityof mechanosensitive calcium channelshas remainedelusive. Molecular identificationis also hamperedby the highly artilicial conditionsrequired to identify stretch-activated ion fluxesby patch-clamp techniques. Removalof the cell wall, isotonicconditions, and suction by the holding electrodecreate conditions where most ion channels (Gustinet al. l99l). would be definedas mechanosensilive Although both mechanisms,stretch-inducedconformationalchanges and stretch-activatedion channels, are often discussed separately,they mi-eht. in fäct. act in concert,as componentsof a so-calledpiasmalemmalreticulum (for a review seePickard2008).This integrativestructurecomprisesadhesiveeomponents (among others arabinogalactan proteinsand wall-associated kinases)that link the plasma membranewith the cell wall, and are also connectedwith mechanosensorycalcium channeis. This structure has been demonstratedand describedfor tobacco BY-2 as a cell biological model system (Gens et al. 2000: Pickard and Fujiki 2005). The plant integrin analogueshave been reported to connect microtubules. plasma membrane, actin filaments and stretch-activated membranechannels(Telewski2006).It is highiy conceivablethat sucha network could act, on the one hand, as a tensegral entity that can convey and focus mechanicalfbrce upon stretch-activating membranechannelsand,simultaneously. transduceforces into conformationalchangestl'at can result in diff'erentialdecoration with associatedproteins that can act as a trigger for signallin-g.The necessityfor stress-focussing is supportedby estimationsof the activation energiesfor mechanosensitive channels(aroundI mN m l. SachsanclMorris 1998)in a range not fär below the lytic tension of plant membranes(around 4 mN m I, Kell and Glaser 1993). If the tensegralcytoskeletonis linked to the cell wall through such integrarive linkers,this shouldbecomemanifestas organizinginfluencesof the cell wall upon the cytoskeleton.This has been observed.For instance,removal o1 the cell wall rendersmicrotubulescold-sensitive in tobaccocells (Akashiet al. 1990).When in the samecellsthe incorporationof UDP-glucoseinto the cell wall was blockedby the herbicideisoxaben(Fisherand Cyr 1993).this impairedthe axialiry of cell expansion,resultingin isodiametriccells and disorderedcorticalarraysol'microtubules.Thus. the mechanicalstrainsproducedby cellulosemicrofibrils ali-sn cortical microtubules,closing a regulatorycircuit betweenthe cell wall and the cytoskeleton.Since expansionis reinforcedin a direction perpendicularto the orientation of microtubulesand microfibrils, fbrces will be generatedparallel to the major strain axis. These forces are then relayed back through the plasma 68 P. Nick membraneupon cortical microtubulesthat are aligned in relation to these strains. Since individual microtubules mutually compete for tubulin heterodimers,and since the number of microfibrils is limited by the quantity of cellulose synthase rosettes,this regulatory circuit should follow the rules of a reaction*diffusion system (Turing 1952) and should therefore be capable of self-organizationand patterning. How could mechanicalstrainsfrom the cell wall causea correspondingalignment of cortical microtubules?The so-calledmicrotubule plus-end tracking proteins (+TIP proteins)seemto play an importantrole in this context.Theseproteins associatewith growing plus endsof microtubulesand form complexesthat control microtubule dynamics, organization and the interaction of microtubules with membranes,organellesand proteins (for a review see Akhmanova and Steinmetz 2008). EB I, a central componentof this complex, is important in searchingfor socalled exploratorymicrotubulesfor intracellularcapturesitesthat are often marked by specific actin structures.For instance, such capture sites are laid down by the preprophaseband and the phragmosomeprior to mitosis and are recognized by exploratory microtubules emanating from the cell poles during telophase (Dhonukshe et al. 2005). Because of this function, several members of +TIP proteins interact with the actin cytoskeleton.Some +TIP proteins,such as adenomatouspolyposiscoli (Moseleyet al. 2007) and CllP-associatedprotein (Tsvetkov et a|.2007), interactwith actin filamentsdirectly, othersinteractthrough the actinbinding formins. A third group, including EB l, interactwith the dynacrincomplex linking the minus-endmicrotubulemotor dynein with actin filaments(for a review seeTimauer and Bierer 2000). EB I binds to microtubuleplus endsat the seamthat joins the tubulin protofilaments(Sandbladet al. 2006) and is therefore a good candidatefor a conformationalmechanosensor.During microtubule catastrophe, the protofilamentsbend outwards,which meansthat they have to be actively tied togetherto sustainmicrotubulegrowth. The +TIP complex,in general,and EB l, in particular, are thereforesubjectto mechanicaltension and must be consideredas primary targets for mechanical strains on microtubules. ln fact, Arabidopsis mutants in members of the EBI family have been found to be touch-insensitive (Bisgroveet al. 2008). Summarizing,in plant cells both stretch-inducedchangesof protein conformation and stretch-activated ion channelsseemto act in concertduring the perception of mechanicalstimuli. The cytoskeletoncan participatein both pathways,either as a stress-focussing susceptorof mechanicalforce upon mechanosensitive ion channels or as a primary sensorthat transducesmechanicalforce into differentialgrowth of microtubule plus ends. Microtubules are endowed with nonlinear dynamics, leadingto phasetransitionsbetweengrowth and catastrophicshrinkage.ln addition, they have to compete for a limited pool of free heterodimers.Microtubules are thereforeideal devicesto amplify the minute inputs from mechanicalstimulation (small deformationsof the perceptivemembranes,changesin the dynamic equilibrium betweenassemblyand disassemblyof microtubulesat the microtubule plus end) into clear and nearly qualitative outputs that can then be processedby downstreamsignalling cascades(for a review seeNick 2008b). P.Nick L:::-.J.rrrrrelationto thesestrains. :r. : ,: rubultn heterodimers,and u'fc --,.rntll\ clf cellulosesynthase l.:iii :.,.J. Lrf a reaction-diffusion .:1.:r.t Lrl self-organization and *.1.. ,-,u\c a corresponding align: i - : : . r h u l ep l u s - e n dt r a c k i n gp r o : ' . : . : .l l l . e o l l t e x tT. h e s ep r o t e i n s '. ::..i :\)r'nteomplexesthat control : n l : : . r , l l \ ) l to f m i C r o t u b U l ews i t h :.r .:: .\khmanovaand Steinmetz \. :. ::r)porll-rflt in searchingfor so. j!-:il:. .rtesthatareOftenmarked -. -.,r:Lrr. sites are laid down by :,,: :., ntitosisand are recognized :hr.rll poles during telophase 1 . 1 1 , ' l r\ c. \ e r a l m e m b e r so f + T I P ,:rr * I IP proteins,suchas adeno1'l-I l'-.r.'ociatedprotein(Tsvetkov . L\tli:s rnteractthroughthe actinn:j:,:- r ri ith the dynactincomplex r * : i r .. r .r i r rl i l a m e n t s( f o r a r e v i e w :,'r-ru,r' plusendsat the seamthat ' 1. x ,rr, und is therefore a good D*: :r: nricrotubulecatastrophe, :r:r ilij\ haveto be activelytied P - :r.:..r ',. in general,and EB I , in e:..: :. .lnrl ntust be consideredas r,r r-.^:,.i:. ln fäct, Arabidopsis ct:. : ..nJ ro be touch-insensitive '-t: c \ . rnLd:of proteinConforma: ,:'11Juringthe perception .:r hothpathways,eitheras :.:. hanosensitive ion chan:- i rnto differentialgrowth ,..rth nonlineardynamics, :-:rr..hrinkage.In addition, .ilin.r\. Microtubulesare . : r r e h a n i c aslt i m u l a t i o n - r r rl h ed l n a m i ce q u i l i b . : t r h c r n i c r o t u b u lpel u s tlr;rr he processedby .,rh Mechanics of the Cytoskeleton 4 69 The Cytoskeleton as a Sensor: Intercellular Sensing It was the loss of buoyancy as a supporting force that drove the evolution of mechanicalintegrationin terrestrialplants.Signalling through a mechanicalsignal is much faster than any diffusion-basedprocessand its velocity equals or even exceedsthat of electric signalling.Moreover, mechanicalintegrationis holistic in nature,sinceit allows the sensingalmostsimultaneouslyof the presenceor absence of building elementseven if they are remote from the sensingcell (as long as they are mechanicallycoupled).The stiffer this mechanicalcoupling, the lessenergy is dissipatedduring signalling. Microtubules are endowed with a high degree of rigidity (Gitteset al. 1993)and thereforerepresentideal transducersfor mechanical integrationeven acrossthe bordersof individual cells. Such microtubule orientations that transcendthe borders of individual cells have been reportedduring phyllotaxis (Hardhamet al. 1980; Hamant et al. 2008). In wounded pea roots, a supracellularalignment of microtubulesheraldscorresponding changesof cell axis and cell divisions such that the wound is efficiently closed (Hush et al. 1990). A curious case of microtubule patterning was discovered in the Arahidopsis mutants spiral, lefty and tortifolia (Furutani et al. 2000; Thitamadeeet aI. 2002; Buschmannet al. 2004).In thesemurants,microtubulesare alignedover many cells in the distal elongationzone of the root (spiral and lefty) or the petiole (tortifolia), accompaniedby twisted growth. The twisted growth phenotypesof thesemutantsareconventionallyexplainedon the basis of uniformly oblique arays of microtubules (and consequentlymicrofibrils). In the spiral, lefty and tortifolia mutants, it is either tubulin itself or microtubule-associated proteins that are affected by these mutations. Moreover, spiral growth can be phenocopiedin the wild type by inhibitors of microtubule assembly(Furutaniet al. 2000). As pointedout earlier,the microtubule-microfibril circuit is endowed with self-amplificationlinked to mutual inhibition. A typical systemicproperty of sucha self-organizingmorphogeneticsystemis an oscillating output (Gierer 1981).Any factorthat altersthe lifetime of microtubuleswill alter the relay times within this feedbackcircuit. Since neighbouringcells are mechanically coupled by tissue tension, even a weak coupling will result in a partial synchronizationof the individual circuits (Campanoniet al. 2003), and the degree of synchronywill dependon the velocity of the feedbackcircuit. Thus, mutationsin an associatedprotein such as the tortifolia gene product (Buschmannet al. 2004), mutationsin tubulin itself, as in caseof lefty (Thitamadeeet al. 2002), or treatment with microtubuleinhibitors (for a review seeHashimotoand Kato 2006)is expected to enhancesynchrony,leading to the observedoscillationsof growth. In contrast, the synchronyshould be reducedwhen microtubule lifetimes are increased,which seemsto be the casefor the mutant radially sw,ollen6 (Banniganet al. 2006), where microtubulearraysare orderedwithin individual cells,but deviatestronglybetween neighbouringcells, suggestingthat supracellularalignmentis affected. The impact of microtubules for mechanointegrationcan be exemplarily studied in the context of gravity responses(see chapter "Mechanical Aspects of P. Nick 10 To compensate Gravity-ControlledGrowth, Developmentand Morphogenesis"). of fbrcethe arrangement optimize plants have to gravity, load by for mechanical mechanical provide optimal they that such in a manner in space bearingelements consumeminimal biomassand are as light as possible. support,but simultaneously only be achievedwhen the arrangementof supportive can This optimization task pattern of mechanicalstrain.Thus, gravity has to be guided by the structuresis has. in addition. to be linked to morphogenesis. it and perceiveclvery efficiently with respectto gravity. the plant will plant is changed a of When the orientation restore the original orientationand thus to way as to a in such respondby bencling (gravitropism). When new organsdevelop,they areofien stress minimizemechanical role (gravimorphosis). When the mechanointegrative gravity to adjustedwith respect separate imponant to gravity, it is to respect with is of the cytoskeleton discussed so-calledsusceptionfrom perceptionin the strict sense."Susception"meanstransformationof physicalenergyinto a differenttype of energythat can be perceivedby the perceptivesystem(Björkman 1988).For instance,the differencein gravitational field strengthbetweenthe two flanksof a misorientedplant would be cefiainly far too small to be sensedby any biochemicalprocess.lt is generallyacceptedthat gravity is first transfomed into mechanicalforce by acting on heavy panicles.the so-called statoliths.Thesestatoliths(as well as their accessorystructures)themselvesare not gravisensitive,but they assistsensingby acting as susceptors. 4.1 Microtubules and Gravitropism For the rhizoid of Chara, experimentsby JohannesBuder ( 1961) demonstratedthat vesiclesfilled with barium sulfate, the Glanzkirperchen,are necessaryand suffitheory For higher plant::,the classicalstarch-statolith cienf for gravisusception. perceptive in the postulated amyloplasts (Nemec 1900; Haberland 1900) that tissues(e.g. root cap or bundle sheathcells) are responsiblefbr the susception of the gravitropic stimulus. A long tradition of experimentationdemonstrated that amyloplastsare necessaryfbr efficient gravitropism.For instance,gravitropic mutants.However, it took almost a sensitivitywas reducedin starch-deficient of centuryuntil it was shown that susception energyby amyloplastsis sufflcient to trigger a curvatureresponse.By using high-gradientmagneticfields, Kuznetsov in inducingbendingin verticallyorientedroots and Hasenstein(1996)succeeded and thus were able to prove very elegantlythat the generationof mechanicalfbrce by statolithsis sufficientfor gravisusception.lt is an irony of sciencehistory that this breakthroughwas not achievedby the elaborateand expensivemicrogravity experimentsin the contextof spaceresearch.but insteadthrough a very cheap.but well-designedground experiment.Thus, in higher plants as well. the primary althoughthe actual by statolithicparticles(the amyloplasts), stimulusis procluced perceptionevent has remainedelusive so far. For the rhizoid o1 Cltara the sedimentationof lhe Glanzlörperchento the lower flank ofthe rhizoid was found to divert vesicleflow towardsthe upperside suchthat p. Nick \ 1 :' -. nL'\l:" ). To compensate . ::rJ ln'angement of force. . . 'r rrlc optimal mechanical .- .:r!i .ircas light as possible. : - . . : r ' , r n s e m e notf s u p p o r t i v e . ; I-hLrs.eravity has to be ' . .nk.,l lo morphogenesis. : ' - , i r ! \ - u | a v i t y ,t h e p l a n t w i l l - n.rl olicntation and thus to : _.rn\ (lc\elop, they are often -" :trr nrcchanointegrativerole : . . 111\ mportant to separate .-' \Ll\ception" means trans' -':i\ thrt can be perceived by ': ,' .lrllcrence gravitational in ' t .i , 'rrlrlhe cerlainly far too ' ' . 1 , ,r t . r ' e p t e dt h a t g r a v i t l i s r r.r\ \ pufiicles, the so-cailed -:.'.1!lLlfcs) themselvesare not --l\l\)l\. r r . r 1 t ) 6l ) d e m o n s t r a t e d t h a t , .r'e necessary and suffi.. -.r, rturch-statolith theory 1 . 1 . 1 . 1r n. t h e p e r c e p t i v e r . r h r c I ' o rt h e s u s c e p t i o n .'. : .:).lrtation demonstrated f ' r i r r ' l r n c e .g r a v i t r o p i c i: ,icrer. it took almost a . . : r \ l , r p l u s l si s s u f f i c i e n t . : i n e t i c f i e l d s ,K u z n e t s o v ' . : r t i c l l l y o r i e n t e dr o o t s ' ' rrt n l e c h a n i c a lf o r c e :-.." l'ri - l\ -- :-' . . Lrl \cience history that . ' r ' i ' t r . i re m i c r o g r a ri t y . : , , 1 r - cah v e r y c h e a p ,b u t '- .1: \\ell. the primary . i . : r l t h o u g ht h e a c t u a l t , t l t t ' t tt o t h e l o w e r L r p p 6 1 - s i 6s lusc ht h a t M e c h a n i c ro l t h e C 1t o s k e l e t t ' n 1l more materialis intussuscepted into the upperflank. resultingin a growth dif'ferential driving downwardbending(Sieversand Schröter197l). This hypothesiswas iaterextendedto negativegravitropismby combiningsedimentationwith a dilferent mode of growth (Hodick 1994).Accordingto this model,the actualperceptionof gravity would rely upon a proximity mechanism.It is doubtful that proximity is used for graviperception in higher plants becauseclassicalstudies(Rawitscher1932) using intermittentstimulalion showedthat perceptioncan occur in the absenceof amyloplastsedimentation.Moreover,dose-responsestudiesemploying centrifugaeven fbr tion have shown that the output (gravitropiccurvature)is dose-dependent stimuli that completely saturateamyloplastsedimentation.Even for the rhizoid of Chara, fbr which the proximity mechanism was originally postulated, it was demonstratedthat strong stimuli that saturatethe sedimentationof the Glan:kir(HenelandFriedrich1973).This suggests perchencanstill be discriminated thatthe actual perceptionof gravity is not basedon proximity, but is basedon the force exertedby the statolithson a mechanosensor suchas stretch-activated ion channels and/orthe cell wall-plasma membrane-cytoskeletoninterfäce. If gravity is perceivednot by proximity but by pressure.this poses a big challengefor the sensingmechanism.Sincegravity is sensedby individualcells (in contrastto the direction of light in phototropism;Buder 1920;Nick and Furuya 1996),the maximal energyavailablefor stimulationis the potentialenergyof the sensingcell. This energybarely exceedsthermalnoise if it is not focussedupon small areas.Theseconsiderations stimulatedresearchon a potentialrole of microtubulesas amplifiersof gravitropicperception.In fäct. gravitropismcan be blocked by antimicrotubulardrugs in the rhizoid of Chcn'o(Hertel and Friedrich 1973) as (Schwuchowet al. 1990:Walker and Sack 1990)or in well as in mossprotonemata coleoptilesof maize(Nick et al. 1991) and rice (Godbol6et al. 2000: Gutjahrand Nick 2006) at concentrationsthat leave the machinery fbr growth and bending essentiallyuntouched.Conversely,when the dynamicsof microtubulesis reduced as a consequence of eithera mutation(Nick et al. 1991'lor treatmentwith taxol,this results in a strong inhibition of gravitropic responses(Nick et al. 1991 Godbol6 et al. 2000: Gutjahr and Nick 2006). The gravitropically induced reorientationof cortical microtubuleshas been observedfor both shoot gravitropism(Nick et al. l99l) and root gravitropism(Blancaflorand Hasenstein1993).In maize coleoptiles, the microtubulesin the epidermal cells of the upper flank of the stimulated organ assumeda longitudinalorientation.whereasthe microtubulesin the lower flank remainedtransverse.By microinjectionof fluorescenttubulin into epidermal cells of intact maize coleoptiles, it was later even possible to demonstratethe gravitropicmicrotubulereorientationin vivo (Himmelspachet al. 1999). The time courseof this responsewas consistentwith a model wheregravitropicstimulation induceda lateralshifi of auxin transporttowardsthe lower or-{anflank and,as a consequence, a depletionof auxin in the upperflank.The microtubularresponse was thought to be primarily by this decreasein auxin concentrationrather than by gravity itself. In maize roots,however,where a similar reorientationwas observed in the cortex(BlancaflorandHasenstein| 993),the time courseof reorientation was found to be slowerthan the changesin erowthrate inducedbv gravitv. i) p. Nick This leadsto the questionof whetherthe gravitropic responseof microtubulesis ,. direct or whethermicrotubulesmerely ."rpo-nd to changesin growth rate.rn fact, it is possibleto induce microtubulereorientation by bendingcoi.optit", *ith manual force (Zandomeniand Schopfer r9g4) - the microtubuleswill then becomelongi_ tudinal in the concaveflank, but remain transverse in the convex flank. To dissect the gravitropic responseand a potentialresponse to changedgrowth rate, microtu_ bule behaviour was followed in coleoptiles that were]..änr"J U], a surgicat adhesivefrom elongating and were kept either in a horizontal orientation (such that a gravitropic stimulation occurreä) or in a vertical orientation (such that growth was inhibited in the absence of a gravitropic stimulus). In this set-up, microtubule reorientation from transverse tJ longitudinal was obse.ved only in the horizontalorientation(Himmerspachand Nick 2001), demons,*iing unequivocally that microtubules,at least in this system, respondedto gravity rather than to t h e i n h i b i r i o no f g r o w r h . 4.2 Microtubules and Gravimorphosis It seemstrivial that roots form at the lower pole of a plant organ, but this is a manifestationof gravimorphosis.Although a considerabi.umouit of phenomeno_ logical work was dedicatedto this probi-em at the tum of the nineteenthcentury (vöchting 1878;Sachslgg0; Goebei r90g), the underlyingmechanismsare still fär from being understood.This is partially'due to the use of adult organs, where polarity has arreadybeenfixed and is haid to invert. However, in germinatingfem spores' the first asymmetric,division that separatesa larger, vacuoratedrhizoid precursorfrom a r-"11:1.u1gdenser tha'us precursorcan be oriented by graviry (Edwardsand Roux 1994).This lirst cell division is clearly of fomative character; when it is rendered symmetric by treatment with antimicrotubular herbicides (vogelmannet al. 1981),the two daughtercells both give rise to thalloid tissue. when this sporeis tilted after the axis Jf th. firrt divisiÄ has beenderermined,the rhizoid will grow in the wrong direction and cannot adjust for this error (Edwards and Roux 1994).prior to division, at the time when the spore is lornpetent to the influence of gravity, a vivid migration of the nucleus towards the lower ltt.t:ilghalf of the sporeis observed.This movem"ent is not a simple sedimentationprocess becauseit is oscillatory and interrupted by short periods of active sign reversal, indicating that the nucleusis tetheredto a Äotive force (Edwardsand Roux r997). The action of antimicrotubular compounds strongly suggeststhat this guiding mechanismis basedon microtubulesthat probabty aügn-,irrr, tne gravity vector, resemblingthe determinationof the grey crescentin amphibian .t ut. 1981), where the dorsoventralaxis is determined "ggiC".nurt by an interplay among gravltydependentsedimentationof yolk particles, spem-induced nucleation of micro_ tubules and self-amprifying alignment of ne*ry formed microtubules that drive cortical rotation (Elinson and Rowning lggg). P. Nick 1 rl: :'. - - j:pfrilS€ of microtubules is r,, - i..:t.rJr ln growth rate. In fact, it t r r;;1.1,',. colr-optileswith manual r-: ':..r...c\ rrill then becomelongi.:\' :. tilr' c()nvex flank. To dissect < ' : - r . . r r r j c tsl r o w t h r a t e . m i c r o t u i:: .1j:t pre\ented by a surgical : ::'. -: it')rirontal orientation (such i : .:rlrctl orientation (such that :!.::,.i. \ttnlulus). In this Set-up, l . ' r : . : . r J r n u i w a s o b s e r v e do n l y i n -r I l'.,lemonstratingunequivo:.\l'. \n!l.rl to -eravity rather than to :s r'.:' ,: .i plant organ, but this is a rn.r.1i:.rblr-amount of phenomeno::.j :-.:'n o1 the nineteenth century LnltJ:.\ iltg mechanismsare still far l,' ::lr u\c o1 adult organs, where r :::i llr rr\ c\ er. in germinating fern i:r:. .r lrtrser. vacuolated rhizoid ,'- -:. ,: .,1n be oriented by gravity r :. -.-..:;.1\ttf fbrmative character; - ::: .:nrinticrotubular herbicides . :\ :: ir\c'rise to thalloid tissue. I . : . , . . . , : r h a : b e e n d e t e r m i n e d ,t h e ßi, i .:.r .r.r tbr this error (Edwards r:..3: ::.J .f ()re is competent to the ' : : - i : ' . u i l e u st o w a r d s t h e l o w e r $.r: -: ..:--.rrla:cclimentationprocess r: t.i: ,.1. ()t tctive sign reversal, < : :, J [:.lri rLrdsand Roux 1997). :.r.- . ..r-jL.:rs that this guiding 1 1 . . .. : t r . . \ l l h t h e g r a v i t y v e c l o r . s -i .: .::rl.r.itreggs (Gerhart et al. .r: - , .: rr.rpl.namong gravity:::. : . _ - : - i n u c l e a t i o no f m i c r o r : - - - - . : : l l ! r ( ) t u b u l e st h a t d r i v e tt I Mechanics of the Cytoskeleton 4.3 13 Microtubules and the Sensing of Gravity Since microtubulesguide the anisotropicdepositionof cellulose in the cell wall, it is not trivial to discriminatetheir function in gravity sensingfrom their role in the responseto gravity during the development of tropistic curvature. When gravitropic bending is inhibited by antimicrotubularagents,this might be caused by a block of either the sensoryfunction or the effectorfunction of microtubules.To discernthesemicrotubularfunctions,the lateral transportof auxin can be usedas a responseupstreamof differential growth. With use of radioactivelylabelledauxin in rice coleoptiles(Godbol6 et al. 2000), lateral auxin transportwas found to be blocked by ethyl-1V-phenylcarbamate, a herbicidethat binds to the carboxy terminus of ü-tubulin and inhibits assemblyof tubulin heterodimersto the growing ends of microtubules(Wiesler etal.2002).Interestingly,Taxol inhibited lateraltransport partially without any inhibition of longitudinal transportof auxin. This indicates that sensorymicrotubuleshaveto be not only present,but, in addition,also dynamic to fulfil their function. The high dynamicsof this sensorymicrotubule population might also explain the extreme sensitivityof gravisensingto low temperaturethat would be difficult to explain otherwise(Taylor and Leopold 1992).The necessityof high microtubular turnover favours a model where microtubules sensegravityinducedforcesactively ratherthan merely acting as gravisusceptors. The gravisensory function of microtubulescan be specificallyblocked by acrylamide (Gutjahr and Nick 2006), a widely used inhibitor of intermediate-filamentfunction in mammalian cells (Eckert and Yeagle 1988). Similar to ethyl-N-phenylcarbamate, acrylamide interrupts a very early step in the gravitropic responsechain, clearly upstreamof auxin redistributionand differential growth. No clear homologuesof intermediate-filamentproteins are known in the plant kingdom, but acrylamide treatmentspecificallydisruptsmicrotubules,leaving actin filaments,for instance, untouched(Gutjahr and Nick 2006). The immediatetarget of acrylamidein mammalian cells seemsto be a kinase that phosphorylateskeratin (Eckert and Yeagle 1988).Since kinasesand phosphatases have been shown to regulatethe organization of plant microtubules(Baskinand Wilson 1997),the inhibition of gravitropism by acrylamidemight be causedby interferencewith the regulatorycircuits activein the highly dynamic microtubule populationresponsiblefor gravisensing. 4.4 Microtubules and Mechanosensing Gravity is not the only source of mechanicalstimulation used to integrateplant architecture.In contrast to terrestrial animals, the cells of land plants are not surroundedby an isotonic medium, but are surounded by a hypotonic medium, with the consequencethat their plasma membrane is under continuous tension from the expanding cytoplasm that is counterbalancedby the cell wall. On the level of organs, considerabletissue tensions develop that can be used for a1 P. Nick when,fbr instance,new organsemergeand will thusgenerate mechanointegration local tension.This phenomenonhas been inlensivelystudiedand modelledfor phyllotaxisby PaulGreenand co-workers.who showedthat modelsof stress-strain patternscouldperfectlypredictthe positionsof incipientprimordia(fbr a revieu'see Green 1980).In the growing meristem,the formation of new primordia is suppressedby the olderprimordia.The tissuetensionpresentin an expandingmeri\tem producesmechanicalstresses resultingfiom bucklingof the precedingprimordia. One of the earliesteventsof primordial initiation is a reorientationof cortical microtubulesthat are perpendicular with respectto the microtubulesof their noncommittedneighbours.This differenceis sharp.but later it is smoothedby a transitionalzoneof cellswith obliquemicrotubules, suchthat eventuallya gradual. progressive chan-ee in microtubularreorientation emergesover severalrows of cells (Hardhamet al. 1980).This phenomenon hasbeenrevisitedrecentlymaking useof microtubulemarkerlineslabelledwith greenfluorescent proteinin the developing shootapexof A. tlnliana (Hamantet al. 2008).wherea feedbackbetweenmicrotubule orientationand organgrowth was demonstrated. Mechanicalmodellingof the expandingshootmeristempredictedthe transcellular patternof rnicrotubuleorientation that was predictedanclobservedto be alignedwith the directionsof maximal slress.By ablationof specificcells in the outermeristemlayer of the meristem,a redistributionof stresswas inducedand modellecl.As expected,this redistribution causeda conespondingredistributionof microtubularorientation. 4.5 Candidatesfor the Underlying Mechanism The sensingof gravity relies on the mechanicalforcessusceptedby the amyloplasts.This would, at first sight, suggestthat the sensingof gravity and the sensingof mechanicalstimuli shouldrun in parallel.This can be testedby antagonisticapplicationof arlificialbendingstressin antagonism to a gravitropicstimulus: it is possibleto separatethe responseof gravity fiom the secondarymechanical stimulus that is induced by the diff'erential growth during gravitropic bending (lkushima and Shimmen 2005). When, under these conditions,the activity of mechanosensitive channelswas suppressed by gadoliniumin hypccotylsof adzuki beans,this suppressed the (mechanicallyinduced)reorientation of microtubulesin the effectortissue,whereasgravitropiccurvatureproceededunaltered(indicating that the microtubulepopulationresidentin the inner tissuesof the apical hook that is responsiblefor gravisensing remainedfunctional).Thus. at leastin this system, mechanosensing is sensitiveto gadolinium,whereasgravisensing is not. Similarly. when the gravitropicbending of maize coleoptileswas inhibited by a surgical adhesive, the gravity-induced reorientation of microtubuleswasnevefiheless developed (Himmelspachand Nick 2001).suggestingdiff-erentsignalchainsfbr gravisensingand mechanosensing. Althoughthe responses of plant cellsto gravityand mechanicalstimuli are generallydiscussedin the contextof stretch-activated ion channels(Ding and Pickard 1993),the proteinconformationparadigmof sensing r I P. Nick :i: . -. -':-r' und will thus generate .iLr.lrctlancl modelled fbr . : . . - . : t : , . r 1l l i r ) d e l so f s t r e s s - S t r a i n - : :-' . : : -. .t. . : l n l r , l ( l t i(rl b r a r e v i e ws e e ,,: nc\\ primordia is sup: r ' r. r nc \ p a n d i n gm e r i s l e m : rhr preceding primordia. -i '..: .rr.'r' it is smoothed by a - ' . . . - : t l h l t e v e n t u a l l ya g r a d u a l , -": -. _-.. rr\ cr SeV€fäl rows of cells - - : .: . . l J r l f c c e n t l ym a k i n g u s e o f :- . - -. .1 lrr'()teinin the developing . . . l h r r c kb e l w e e nm i c r o l u - : : : : \ 1 . ' . h u n i c a lm o d e l l i n g o f t h e . -. .. :-.,11.'t'n of rnicrotubule orienr,' : tlt rhe directions of maximal :' . ' . l . r rc r o f t h e m e r i s t e m ,a .: i- -.\ |e cted. this redistribution ^ . . . . , :l i n l a t i o n . 'c hani snt - . . u \ e c p t e db y t h e a m y l o .-:r.in! of gravity and the . .iin Lretestedby antago. l\Jit sravitropicstimulus; ' :.-' .cr'ondary mechanical :..: n.r gravitropicbending . , : r ( i r l i ( ) n tsh. e a c t i v i t yo f rn hr pocotylsof adzuki ' . r r,' n , ' l 'n r i c r o l u b u l e s in . :-'.1LrnrLlrered (indicating . - . r r 1t h c a p i c a lh o o kt h a t . . , 1l t u s [ i n t h i s s y s t e m . r . r r i i . n o t .S i m i l u r l l . . r h r h i r e cbl y a s u r g i c a l . - . .. i . n . \ e r t h e l e sdse v e l ' -n,rl chlins for grav' .,'r.tll: to gravity and I : : ' 1l .l - u c t i v a t ei o dn : .,t.r,li!n o tf s e n s i n g M e c h a n i c so f t h e C y t o s k e l e t o n 75 should not be neglected.This is emphasizedby the phenotype ol Arabiclopsis mutants,wherethe microtubuleplus-endprotein EBI is aff'ected(Bisgroveet al. 2008).ln thesemutants,both the initiationof gravitropiccurvatureand a speciltc touch-inducedwaving of roots on inclined agar plateswere affected.Although our knowled-ee of the primary eventsof mechanosensing and gravisensing in plantsis extremelylimited.it is clearevenat this stagethal the rolesof microtubulesmight ditfer qualitatively.[n mechanosensin_e, microtubulesseem to act as susceptor structuresthat focus defbrmation stress towards ion channels. In contrast. in gravisensing. the necessityfbr high dynamicsand dimer turnoverfavoursa direct sensoryrole of microtubules. Thus,naturemight utilize both mechanisms simultaneouslyto sense(andpossiblyto discriminate)differentstimuli.The challengefor future researchin this field will be to design experimentalapproacheswith clear outputsbasedon clear conceptsof the sensingmechanism.Only in a secondstep will it becone possibleto defineand testmolecularand cellularcandidates. 5 The Cytoskeletonas a Sensor:Intracellular Sensing The previoussectiondealtwith the mechanicalintegrationof individualcellsinto a coordinatedresponseof the entire organ. However, mechanosensingis also used to integratethe diff-erentcomponentsof a cell into an individual.This becomes evident as redistributionof organellesin responseto mechanicalstimulation(thigmomorphogenesis;chapter "Mechanical Force Responsesof Plant Cells and Plants"),but also involvesresponses that are lessevident.such as the adaptation (chapter"Osmosensing") to cold, the inductionof plant defence.osmoregulation and the regulationof division symmetry.The fundamentalrole of intracellular mechanosensinghas emergedin recent years.but its full impact is still far fiom beingrecognized. 5.1 Thigmomorphogenesis Morphologicalresponses to mechanicalstimulationhave been demonstrated not only on the supracellularlevel. but also on the sub-cellularlevel. When I'ern protonemataare squeezedby a needle,chloroplastsavoid the site of contact (Sato et al. 1999).In epidermalcells(Kennardand Cleary 1997)or in suspension cellsof parsley(Gus-Mayeret al. 1998)sucha localpressure can inducenuclearmovement towardsthe contactpoint. When regeneratingprotoplastsor cells are challengedby eithermild centrifugation or touch.the axisof cell divisionis alignedwith the lbrce vector(Lintilhacand Vesecky1984:Wymer et al. 1996:Zhot et al. 2007).Using tension-freeprotoplasts.Wymer et al. (1996) aligned microtubulesby a shorl centrifugationand thus orientedthe axis of cell expansionin a direction perpendicularto the forcevector.They usedthis systemto demonstrate a role of microtubules P. Nick 76 To separatethis sensoryrole from the microtubularfunction in in mechanosensing. cell-wall synthesis,microtubuleswere eliminatedtransientlyduring the application of force by amiprophos-methyland allowed to recover by washing out the herbicide, suchthat cellulosesynthesiscould occur and thus the cell axis could develop. When this transient microtubule elimination was performed either immediately before or immediately after the centrifugation,the alignment of cell division was not observed.ln a control, microtubuleswere eliminated subsequentto the centrifugation, which did not impair the alignment of the cell axis by the mechanical stimulus.This demonstratedclearly that microtubulesare necessaryfor the sensing walled cells of of this mechanicalstimulus. A recent study in agarose-embedded Chrl-santhemum(Zhou et al. 2001) extendsthese findings by the interactionwith the cell-wall cytoplasmiccontinuum.Here,cell divisionswere alignedby compression force (Lintilhac and Vesecky 1984). When microtubules were removed by oryzalin prior to the treatmentor when the cell-wall cytoplasmic continuum was impaired by treatmentwith RGD peptides,this alignmentresponsewas interrupted. ln contrast.elimination of actin filamentsby cytochalasinB was not effective. 5.2 Cold Sensing The sensing of temperature must occur cell-autonomously.This is generally ascribed to a reduced fluidity of membranesthat will alter the activity of ion channelsor the balanceof metabolites(Lyons 1973).For instance,overexpression has been shown repeatedlyto modify chilling sensitivity in plants of desaturases (Murata et al. 1992). Since microtubulesdisassemblein the cold, they have long been discussedas alternativesensorsfor low temperatures.ln fact, when microtubuleswere manipulatedpharmacologically,this was accompaniedby changesin cold hardiness(Kerr and Carter 1990). Microtubule disassemblyof plants and of animalsin the cold differ dependingon the type of organism.Whereasmammalian microtubules disassembleat temperaturesbelow 20"C, the microtubules from poikilothermic animals remain intact far below that temperature(Modig et al. 1994).ln plants, the cold stability of microtubulesis generally more pronounced than in animals, reflecting the higher developmentalplasticity. However, the critical temperaturewheremicrotubuledisassemblyoccursvariesbetweendifferent plant species,which is correlatedwith differencesin chilling sensitivity(Jian et al. 1989). The close correlation between microtubular cold sensitivity and general chilling sensitivity is supportedby the observationthat abscisicacid, a hormonal inducer of cold hardiness(Holubowicz and Boe 1969; lrving 1969; Rikin et al. 1975; Rikin and Richmond 1916),can stabilize cortical microtubulesagainstlow temperature (Sakiyama and Shibaoka 1990; Wang and Nick 2001). Tobacco mutants, where microtubules are more cold-stable owing to expressionof an activation tag, are endowedwith cold-resistantleaf expansion(Ahad et al. 2003). Conversely,destabilizationof microtubulesby assemblyblockers such as colchicine and podophyllotoxinincreasedthe chilling sensitivityofcotton seedlings,and t I P. Nick . :: : . li"i nlcrotubularfunctionin :.: ::.:: .i,'nrl\ duringthe application :i- ,:: hr *ashing out the herbi,:rr if .... rhc ccll axis could develop. s j\ -\r'''\rrnredeither immediately : i : . , . : * n r r r conlt' c e l ld i v i s i o nw a s r.i:'.:r.:lJrl\ubsequentto the centri,': :::: .tll axis by the mechanical for the sensing ua-.r. .:r. necessary i i . : : , , . i - e n r b e d d ew d a l l e dc e l l so f :.. :rr!irngsby the interactionwith j,,, .;1r11r *ere alignedby compres:r'.r.lr)tubules were removedby .n was [ . - . - . , .. r t o p l a s m i c o n t i n u u m was interrupted. :. : :::::r:nt fesponse , : , \ : ' . . r . . r \ iB n w a sn o t e f f e c t i v e . [-r-: 'n()nror.lsly. This is generally r.rr-:l:rrll alter the activity of ion . - - I I - ( , ri n s t a n c eo.v e r e x p r e s s i o n r'..r:: . .hilling sensitivityin plants {':.^.r rn the cold, they have long rs:.:-L:.rture s. ln fact, when micro. . . , . Lr. . r . eo m p a n i e db y c h a n g e isn -^.. -. .ir.r\\ernblyof plantsand of -' : : -.1ili\m.Whereasmammalian ." - ('. the microtubules from r'niperature (Modig et al. - :rne rally more pronounced rf:-- -.: r.: plir\ticity. However, the ria... - - !ri\ \ aries between different - : . . i r n g s e n s i t i v i t y( J i a n e t a l . !s. -5-.-:r -,.J 'ensitivity and general \-..- il: : ::'..r:.rl,scisicacid, a hormonal i.-lc " ' lrring 1969; Rikin et al. - - . : : I r . r ( ) t u b u l easg a i n slto w - . , : ' . 1\ i c k 2 0 0 1 ) . T o b a c c o \f, .: r 1..3i: . i \ \ : . . l n ! t o e x p r e s s i o no f a n ' : - . , n \ r ( ) rtlA h a d e t a l . 2 0 0 3 ) . ^ .. hirrckers such as colchi. : . L r r. o t t o n s e e d l i n g s a , nd I Mechanics of the Cvtoskeleton this effect could be rescued by addition of abscisic acid (Rikin et al. 1980). Conversely,gibberellin,a hormonethat hasbeenshownin severalspeciesto reduce cold hardiness(Rikin et al. l9l5; lrving and Lanphear 1968), renders corrical microtubulesmore cold-susceptible(Akashi and Shibaoka1987). It is possibleto increasethe cold resistanceof an otherwise chilling-sensitive speciesby pre-cultivationat moderatelycool temperature.Cold hardeningcan be detectedon the level of microtubules as well. Microtubules of cold-acclimated spinachmesophyllcells copedbetterwith the consequences of a freeze-thawcycle (Bartolo and carter 1991a).Microtubulesare not only the targetof cold stress,they seem,in addition, to participatein cold sensingitself, triggering a chain of events that culminates in increasedcold hardiness.when microtubule disassemblyis suppressedby Taxol, this can suppresscold hardening (Ken and Carter 1990; Bartolo and Carter 1991b).This indicatesthat microtubuleshave to disassemble to a certaindegreeto trigger cold hardening.To test this hypothesis,cold hardening was followed in three cultivars of winter wheat that differed in freezing tolerance (Abdrakhamanovaet al. 2003). During cultivation at 4"C, the growth rate of roots recoveredprogressivelyas a manifestationof cold hardening.In parallel,the roots acquiredprogressiveresistanceto a challengingfreezingshock(-7'C) which would impair growth irreversiblyin non-acclimatedroots. when microtubuleswere monitored during cold hardening, a rapid, but transient partial disassembly was observedin cultivars that were freezing-tolerant,but not in a cultivar that was freezing-sensitive.However, when a transientdisassemblywas artificially generated by a pulse treatment with the antimicrotubularherbicide pronamide in the sensitivecultivar, this induced freezing tolerance.This demonstratesthat a transient,prutial disassemblyof microtubulesis necessaryand sufficientto trigger cold hardening,suggestingthat microtubulesact as "thermometers". Similar to mechanosensingand gravisensing,this leads to the question of whether microtubulesact as susceptorsor as true receptorsfor low temperature. The primary signal for cold perceptionis thought be increasedmembranerigidity (Los and Murata 2004; Sangwanet aI.2001). For insrance,the input of low temperaturecan be mimicked by chemical compoundsthat increaserigidity, such as dimethyl sulfoxide, whereasbenzyl alcohol, a compound that increasesmembrane fluidity, can block cold signalling (Sangwan et al. 2001). With use of aequorinas a reporterin transgenicplants,rapid and transientincreasesof intracellular calcium levels in responseto a cold shock were demonstratedby monitoring changesof bioluminescence(Knight et al. 1991). Pharmacologicaldata (Monroy et al. 1993) confirmed that this calcium peak is nor only a by-product of the cold response,but is also necessaryto trigger cold acclimation.This peak is generated throughcalcium channelsin conjunctionwith calmodulin.Calcium and calmodulin in tum are intimately linked to microtubule dynamics.Immunocytochemicaldata show that microtubulesare decoratedwith calmodulindependingon the concentration of calcium (Fisherand Cyr 1993).Ir was further suggestedrharthe dynamicsof microtubulesis regulatedvia a calmodulin-sensitiveinteractionbetweenmicrotubules and microtubule-associated proteins such as the bundling protein EF-lr (Durso and cyr 1994).However,the interactioncould be evenmore direct.because 78 P. Nick cleavageof the carboxy terminusof maize tubulin was shown to renclermicrotubulesresistantto both low temperature and calcium(Bokroset al. 1996).When the releaseo1'calciumfiom intracellularpools was blocked by treatmentwith lithium, an inhibitor of polyphosphoinositide turnover,this resultedin increasecl cold stabilityof microtubulesin spinachmesophyll(BartoloandCarter1992).wirh use of a cold-responsive reportersystemit was demonstrated that disassembly of microtubulesby oryzalin or treatmentwith the calcium ionophoreA23 187 could mimic the ef'tectof low temperature,whereasthe calcium channelinhibitor gadolinium or suppression of microtubuledisassembly by taxolpreventedthe activation of this promoterby low temperature(Sangwanet al.200l). Thesedata fävour a model wheremicrotubulesact as receptorsthat limit the permeabilityof calcium channelsthat are triggered by membranerigidification. when microtubulesfunction as modulatorsof calcium channelactivity and when microtubuleintegrityis regulatedthroughcalcium/calmodulin, this would set-upa regulatorycircuitcapable of self-amplilication: Stablemicrotubulesthat limit the activityof cold-inducecl voltage-dependent calcium channelswould. upon disassernbly, releasethis constraintand this would elevatethe activityof the channels.resultingin an increased influx of caicium.This calciuminflux,in tum, would resultin furtherclisintegration of the microtubularcytoskeletonand thus trigger by this positive-f'eedback the influx of additionalcalcium. A very small initial calcium influx might thus be amplifiedinto a strong signal that can be easily processedby the activationof calcium-dependent signallingcascades. The resultingsignallingcascadewill activate colclhardeningas an adaptiveresponseto cold stress.Interestingly.microtubules will be renderedcold-stableas a consequence of this coid hardening (Pihakaski-Maunsbach and Puhakainen1995; Abdrakhamanovaer al. 2003), which. in turn, should result in reducedactivity of the calcium channelsthat respondto membranerigidification.Thus, microtubuleswould not only enclow cold sensingwith high sensitivity,but, in acdition.would also endow it with the ability to downregulatesensitivityupon prolongedstimulation.a key requirement lbr any biological sensoryprocess. 5.3 Plant Defence The interactionof pathogensand their hostsis subjectto an evolutionaryarmsrace. wherethe pathogensdevelopvariousstrategies to circumventor suppress det'ence responsesof the host, whereasthe host developsvarious strategiesto senseand attack the invading pathogenor its effector molecules.Cellular responsesto elicitorsincludeformationol cell-wall papillaearoundsitesof pathogenpenetralion. The fomation of these papillae is preceded by a reorganizationof the cytoskeletoncausinga redistributionof vesicletraffic and a cytoplasmicaggregation towards the penetralionsite (for reviews see Takemoto and Hardham 2004: Kobayashiand Kobayashi2008),and a somewhatslowermigrationof rhe nucleus (fbr a review seeSchmelzer2002). By localizedmechanicalstimulationof parsley P. Nick f-. . . . . . . h r r u n t o r e n d e rm t c r o l J , ' k r o se t a l ' 1 9 9 6 ) W . hen - ' . , r t k c d b y t r e a t m e nw t ith . -.: rhi: resultedin increased i i . , : t L r iior n dC a r t e r1 9 9 2 )W . ith ' r i . l | l t e d t h a t d i s a s s e m b loyf - . . : : rr ( ) n o p h o rAe2 3 1 8 7c o u l d .. - nrnt ehannelinhibitorgado-. . ..,rrrl plsvsJlted the activation . . l r ) () l i . T h e s ed a t af ä v o u ra r :ll. permeabilityof calcium : r'L\\'hen microtubulesfunc: ,.hL'ltrtricrotubule integrityis r:\ .,i Lrp1 regulatory circuit caparl thc ilctivity of cold-induced .: -.r..entbly, release this con- L - . . . : . : r , ' r \r.e s u l t i n gi n a n i n c r e a s e d : . . r r l l i n I ' u r r h ed r isintegration '.,. rhrs positive-feedback the -., .iLun influx might thus be ' ,,.;'::erl by the activation of ' - . r - g n l l l i n - sc a s c a d e will acti.t !.\\. Interestingll.micro-...Jr. j of this cold hardening '..: .rt'hurnanova et al. 2003), . rhc calcium channelsthat '.. .. !.\ riould not only endow r:. l _ - ' Lrl.lrrlso endow it with the . , . . t t i o l la. k e r r e q u i r e m e n t ' - \ ( ) l u t i o n a rayr m sr a c c . '.rnt of suppressdef-ence \; \-: F.: i . .t|ute-eies to senseand - C e l l u l a rr e s p o n s etso l-. ()1'pathogenpenetra.. r'.()rganization o1' the : -rlrrplu:mia c ggrega' ' ' . r n . lH a r d h a m2 0 0 4 ; ' : . r r i r t l o no f t h en u c l e u s . . , . l r r u l a t i 0 no f p a r s l e y Mechanics of the Cytoskeleton 19 cells, it was possibleto partially mimic an attack by Ph,-tophthoraso.jaeand to induceseveralaspectsof a non-hostresistance, includingnuclearmigration.cytoplasmic reorganization,formation of reactiveoxygen speciesand the induction of (Gus-Mayeret al. 1998).In contrast.localizedappliseveraldefence-related -qenes cation of the corespondingelicitor (pep-13)failed to induce the morphologicai changes,althoughit induceclthe full setof defence-related genesand the formation of reactiveoxygenspecies.Interestingly, the elicitorcompletelyinhibitedcytoplasmic aggregationand nuclearmigration in responseto the mechanicalstimulus. Since pep-13 inducesin this systemthe activity of a mechanosensitive calcium channel(Zimmermannet al. 1997).ftseemsthat chemicaland mechanicalsignalling converge during the cytoskeletalresponseto pathogen attack. Neither the mechanicalstimulus nor the elicitor nor their combinationwas able to induce hypersensitive cell deathin theseexperiments, leadingthe investigators to conclude that additional chemical signals are required to obtain the complete pathogen response. This suggests an interactionbetweenmicrotubulesand mechanosensitive ion channelsthat are imDortantlbr the induction of defence. 5.4 Osmoregulation The ability to maintainionic balancerepresents a basiccapacityof all living beings. Prokaryoteshave already developed osmoregulation.In plants, the mechanical tensionsproducedin the context of an expandingtissue have to be balanced by osmoticpressure(chapter"Osmosensing").Microtubulesseemto be directly involved in osmoadaptation. By applicationof osmotic stressto root tips of Tritit-um turgidLrm,microtubuleswere induced to disassembleand to reorganize into massivebundles(Komis et al. 2002).when the fbrmationof theseso-called macrotubuies was suppressed by treatmentwith oryzalin,the protoplastswere no longer able to adapt to osmotic stressby controlled swelling and perished.A pharmacological study (Komis et al. 2006) revealedthat inhibitorsof phospholipaseD. suchas butan-1-oland N-acetylethanolamine. suppressed osmoticadaptation as well as the formationof the macrotubules. In contrast,phosphatidicacid.a product of the action of phospholipaseD, enhancedosmoadaptationand macrotubule lbrmation and was able to overcomethe inhibitory effect of butan-l-ol. Theseobservationsdemonstratethat the microtubuleresponse(formationof macrotubules)is essentialfor osmoadaptation, and that signallingthroughphospholipase D actsupstreamof microtubulesin this response. 5.5 DivisionSymmetry A homologueof the bacterialmechanosensitive channelMscS.MSL3, localizedin discretepatchesin the plastidenvelopeand co-localizedwith the plastiddivision 80 P. Nick factor MinE, indicating an interaction with plastid division (Vitha et al. 2003). When this bona fide channel was mutated,this resultedin chloroplaststhat were irregular in size, shapeand partially number. Thus, these channelsregulatemorphogenesisand developmentof plastids,suggestinga functional shift from osmoregulationtowardsregulationof plastid morphogenesis.However, it is not only in the symmetry of organelledivision where mechanosensingseemsto play a role. Mechanosensingseemsto be the integratorthat allows the nucleusto determinethe correct position prior to mitosis - this premitotic nuclear movement is an active processand is driven by the cytoskeleton.It will detemine the symmetry of the ensuing cell division and thus the basic morphology of the prospectivedaughter cells. The discovery that overexpressionor knockout of the plant-specifickinesin motor KCHI (Frey et al. 2010), which binds to actin filaments,retardsor acceleratespremitotic nuclearpositioning,indicatesthat cytoskeletaltensegrityis used to determinethe correct position of the nucleus.Two principal modesare conceivable that are not necessarilymutually exclusive.Microtubules and actin filaments might transmit forces that are generatedby the KCHI motor at the perinuclear contact sitesto the cortex such that the nucleusis either pulled or pushed,or both (Fig. 2c, d). Alternatively, KCHI might simply anchor the perinuclearnetwork at the cell cortex and move the nucleus by mutual sliding of actin filaments and microtubules in the cortical cytoplasm.From studiesin yeast, filamentousfungi and a variety of animal cells,the molecularmechanismsthat orient and move nuclei were found to be moderatelyconservedand involve as key playersdynein,dynactin and otherproteinsat the plus endsof astralmicrotubules,mediatinginteractionwith the cell cortex and actin filaments (Morris 2003; Yamamoto and Hiraoka 2003). Both repulsiveand attractiveforcesare generatedby a combinationof microtubule polymerizationand de-polymerizationevents,complementedby dynein-mediated sliding of microtubulesalong the cell cortex (Adamesand Cooper2000). In plants, which lack dynein and its associatedproteins (Lawrenceet al. 2001), the mechanisms for nuclear movement must involve fundamentallydifferent players that are able to interact with both premitotic microtubulesand actin filaments.Could KCH proteinsbe thesemissing links as functional homologuesof dyneinsby anchoring minus-end-directedmotor activity to the cortex? 6 EvolutionaryPerspective:The Cytoskeleton as a Central Integrator This chaptersummarizedevidencefor a cytoskeletalfunction in tensegralintegration on both the organismaland the cellular level. We are still far from understanding the molecular set-up of tensegral sensing.But even at this stage, the first differencesbetweenthe different stimulusqualitieshave emerged. For mechanoperception, microtubulesseemto interactwith stretch-activated ion channels,probably focussingminute deformationsof the membraneor changesin P. Nick .lrrr.ron (Vitha et al. 2003). .:. :J....1r(ltn chloroplaststhat were I i .-.. '.ttire channels regulate more . : l :': .1 :L[tcltonal shift from osmoi : ' . : ' . r . .H o w e v e r , i t i s n o t o n l y i n i:'. ,.Jll\lllg seems to play a role. ! g-. .,.. rhe nucleus to determine the r , : : , : - . r r i r f n l o v e m e n ti s a n a c l i v e li i.. .tJl.ntrlne the symmetry of the )., _,. ()t the prospective daughter Ir\.r ,.rl ,)1 the plant-Specifickinesin : . ' , r , : - i t l t l a m e n t s ,r e t a r d s o r a c c e l r li..:i .\rLr:keletal tensegrity is used . I . . ' n r r r r ei p a l m o d e s a r e c o n c e i v - \ 1:.r,,tubulesand actin filaments f'('llI motor at the perinuclear . :. r.lltCf pulledor hcr puiled pushed,or or pushecl, or both both ia i r:1.:t(\r the perinuclear network at r : - : . . . r r l r n go f a c t i n f i l a m e n t sa n d .:-.:.ii. in veast,filamentousfungi ' : : : r r . : : t \ t h a to r i e n ta n d m o v en u C l e i ,r.\ :' ..\ Nc\ playersdynein,dynactin ,,1-r,..r'. ntediatinginteractionwith r 'i. \ .rnlulnotoand Hiraoka 2003). 3 J ' . . , ! ( ) n t b i n a t i oonf m i c r o t u b u l e -.,:r.:-.Jrltented by dynein-mediated .i:: .'. .rntlCooper2000).In plants, L: ",:-':',.i.'t al. 2001).the mechan:::'.::ri.,.lrditl'erentplayersthat are .:r..r.:.tut filaments.Could KCH :lJ\ ()t dyneinsby anchoring rtoskeleton . . . : . . . . : , , t l r ) l li n t e n s e g r a li n t e g r a 'ri. 3- -.:: .till far from understandI b- -..-'n ar this stage,the first i:::. '.:.: l:llCfged. . { ' : : 1 i - . - - : . r r t h : t r e t C h - a C t i v a t ei od n r(.-. ::.r :llinlbrane or changes in Mechanics of the Cytoskeleton 8l membranefluidity towardsspecificmembraneareas.Becausethe demonstrationof mechanosensitivityby patch-clampexperimentsis experimentallyvery problematic and prone to artefacts(Gustin et al. l99l), the molecular identity of stretchactivatedchannelshas remainedelusive in plants.When mechanosensitivityis not an intrinsic propertyof suchchannels,but is conferredby accessorystructures(such as cytoskeletaltensegrity), the identification of these channels will go beyond simple expressionin heterologoussystems(such as frog oocytes),and will require the reconstitutionof the entire structure,i.e. it will rely on syntheticbiology. The situation might be different for the sensingof gravity. Here microtubules themselvescould act as primary sensors.The findingsof the few experimentswhere the involvementof ion channelshas been addressedexperimentally(Ikushima and Shimmen 2005) suggest that these channels might be dispensablefor gravity sensing.The necessityof microtubule tumover in the sensingof gravity indicates a true perceptivefunction ratherthan stimulus susception. The sensingof cold seemsto representa third mechanism,where the gating of cold-sensitivechannels(which probably respondto membranerigidity as an input) is limited by microtubules.when these microtubulesdisassemblein responseto cold, the constraintsupon the activity ofthe ion channelswill be releasedsuchthat calcium can enter, which will facilitate, through interaction with calmoclulin, further disassemblyof microtubulesand thus trigger a positive-feedbackloop. In this systemmicrotubuleswould play a dual function - first as a perceptivedevice and later as accessorystructuresfor the perceptivechannels. why is the cytoskeleton central for mechanical integration? The reason is probably linked to the innate properties of cytoskeletal dynamics that render microtubulesand actin filamentsideal for the sensingof minute and noisy inputs. These dynamics are nonlinear and endowed with autocatalyticproperties.In the cell, the abundanceof monomersis limited, which meansthat different polymers compete for the incorporation of free monomcrs. For instance,in all organisms investigatedso far, tubulin synthesisis tightly regulatedby an elaboratesystemof transcriptionaland post-transcriptionalcontrols, probably to avoid the accumulation of (highly toxic) supemumerousfree heterodimers(for a review seeBreviario 2008). Although the term "cytoskeleton" was coined in the model of a rigid framework that stabilizesthe structureof a cell, such associationsare far from reality. The half+ime of a plant microtubule,for example,has beenestimatedto be in the range of 30-60 s (Hush et al. 1990). Therefore, it is more appropriateto conceive of microtubules and actin filaments as statesof dynamic equilibrium between assemblyand disassemblyof monomers.It is this dynamic equilibrium that provides the major source for the characteristicnonlinearity of cytoskeletal dynamics. Interestingly,the relationbetweenassemblyand disassemblyis practicallynever balanced-one always dominatesover its antagonist.This statementis valid in both spaceand time: in space,becausedimer addition and dispersaldefine a distinct polarity of eachindividual cytoskeletalpolymer, with dimer addition dominatingat the plus end and dimer dissociationdominating at the minus end; in time, because eachpolymer can switch betweena growing state,when dimer addition at the plus t32 P. Nick end predominatesover dimer dissociationat the minus end, and a shrinking state. when dimer dissociationat the minus end exceedsdimer additionat the plus end. The switch between both statesis so swili and dramatic that it has been termed "microtubule catastrophe".These conversionsclependon associatedproteins that can increaseor decreasethe frequencyof transitionbetweengrowth and shrinkage. Becauseof its nonlineargrowth, the cytoskeletonis an ideal tool for developmental patterning.This has been exemplarily shown fbr the induction of the grey crescentin developingfrog eggs.This manifestationof dorsoventralityis produced in an epigeneticprocess,where a gravity-dependentgradient of developmental determinants in the centralyolk interactswith a second.displaced.gradientin the egg cortex(Gerhartet al. 198l). The displacement of the egg coftex is driven by microtubulesand is triggeredby the penetrationof the sperm.The sperm induces the nucleationof microtubulesthat act as tracksfbr a kinesin-drivenmovement (Elinsonand Rowning 1988).The movement,in turn, triggersshearfbrces that align the nucleationof additionalmicrotubulesin a directionparallelto the movement, whereasdeviant microtubulesmore fiequently undergocatastrophictransitions. The resultingnet alignmentof tracksincreases the efficiencyof ntovement andthusthealigningforce.This culminatesin a rapidrotationof thecorticalplasma in a direction fiom the sperm towards the more remote equatorof the egg. This movementwill then causean overlapof uppercortex with a small region of the lower core and eventuallytrigger inductiveeventsthat lay down the Spemann organizer. The combinationof nonlinear,autocatalyticdynamic statesof individual cytoskeletal polymers with the tight control of free monomers accentuatingmutual competition generatessystempropertiesthat are highly relevant lbr sensoryprocesses.Microtubules and actin filaments fullil all formal criteria of a reactitlndifTusionsystem(Turing 1952).This meansthat they can be understoodas ideal pattern generatorsthat are able to produce qrralitativelyclear, neat outputs liom minute and highly noisecontaminatedinputs.One can model how owing to their innate dynamic properties microtubules will spontaneouslyself-organizein responseto even a weak external factor such as gravity or mechanical fields (Tabonyet al. 2004).It thus seemsthat naturehasmadeampleuseof theseunique molecular propertiesto builcl sensorysystemsthat are both sensitiveand robust againststochastic noiseandcanbe usedto integrateevenminutemechanicalstrains on the level of individualceils as well as on the level of entireorsans. References A b d r a k h a m a n o v aA , W a n g Q Y . K h o k h l o v a L . N i c k P 1 2 0 0 3 )l s m i c r o t u b u l ea s s e m b l ya t r i g g e rf b r c o l d a c c l i m a t i o n ?P l a n t C e l l P h y s i o l 4 4 6 7 6 - 6 8 6 Adames NR, Cooper JA (2000) Microtubule interactions with the cell cortex causing nuclear m o v e m e n t si n S a c c h a r o m y c e sc e r e v i s i a e J. C e l l B i o l 1 , 1 9 : t i 6 3 - 8 7 . 1 Ahad A. Wolf J. Nick P (2003) Activation-tagged tobacco mutants that are tolerant to anrimicrot u b u l a r h e r b i c i d e sa r e c r o s s - r e s i s t a nt to c h i l l i n g s t r e s s .T r a n s g e n i cR e s l 2 : 6 1 - 5 - 6 2 9 P. Nick rJ -. - .. ,'ntl. uncl a shrinking state, ' -': .rrirlitionat the plus end. .:::. tllirt it has been termed .: , ,n rr::ociated proteins that -': , rl'n grorvth and shrinkage. . .rn ideal tool for develop' ': r l r . i r r d u c t i o no f t h e g r e y . , l \ r r ' \ ( ) \ ' e n t r a l i ti ys p r o d u c e d : :r'rrrlientof developmental : r . i .. i i s p l a c e d .g r a d i e n ti n t h e lltc egg cortex is driven by '..' .Pcr.nr.The spem induces : .r i.incsin-driven movement .::r. trigsers shearforces that : : - - t i ( ) n p a r a l l e lt o t h e m o v e . . r .l' c l r t r c a l a s t r o p h i ct r a n s i ' litr L'lficiency of movement : rl.rlr()ltof the cortical plasma ,lJ .quator of the egg. This ' - ',rrlh a small region of the in.Lt Iav clown the Spemann ' r, \ti-rtcsof individual cytor . ' , n r c l ' \a c c e n t u a t i n gm u t u a l : .i lelcrant for sensory pro. fi : ::r.rl eriteria of a reaction-,in bc understood as ideal -' ', ilerrr. neat outputs from : : r t r t l eh l ow owing to their ' r . ' ,' r r . l ) s e l l - - o r g a n i z ei n - . .ll\ ()r mechanical fields . : . . 1 \ . t r' : eo l -l h e s eu n i q u e '.,\th \ensitive and robust . .i l r u t cn r e c h a n i c aslt r a i n s -:tli|corgans. t i , r : s e m b l ya t r i g g e rf b r , ' r r l L ' \ c a u s i n - qn u c l e a r r'. tirlerant to antimicro. ll:615-629 M e c h a n i c : o l -: h ü C ) t o \ k e l e t o n 83 A k a s h i T . S h i b a o k aH ( 1 9 8 7 ) E f f ' e c t so 1 ' , e i b b e r e l l i n o n t h e a m a n g e r n e nat n d t h e c o l d s t a b i l i t y o l ' cortical microtubules in epidermal cells of pea internodes. Plant Cell Physiol 2tl:339-3,18 A k a s h i T , K a w a s a k iS . S h i b a o k aH ( 1 9 9 0 ) S t a b i l i z a t i o no f c o r t i c a l m i c r o t u b u l e sb y t h e c e l l w a l l i n c u l t u r e d t o b a c c oc e l l s . E f f e c t o i e x l e n s i n o n t h e c o l d s t a b i l i t y o f c o r t i c a l m i c r o t u b u l e s .P l a n t a 1 8 2 : 3 6 33 6 9 Akhmanova ,4. Sleinmetz MO (20013)Tracking the ends: a dynamic protein network controls the f ä t e o f m i c r o t u b u l et i p s . N a t R e v M o l C e l l B i o l 9 : 3 0 9 - 3 2 2 B a l u i k a F , H l a v a ö k a A ( 2 0 0 - 5 )P l a n t t b r m i n s c o r n e o f a g e : s o r n e t h i n gs p e c i a l a b o u t c r o s s - w a l l s . New Phytol 168:499--503 B a l u S k aF , J a s i k J . E d e l m a n n H G . S a l a j o v äT . V o l k m a n n D ( 2 0 0 1 ) L a t r u n c u l i n B - i n d u c e d p l a n r d w a r f i s m : p l a n t c e l l e l o n g a t i o ni s F - a c t i n , d e p e n d e n tD. e v B i o l 2 3 1 : l l 3 - 1 2 : l Baluika F. Samaj J, Wojtaszek P. Volkmann D. Menzel D (2003) Cytoskeleton-plasma rnemb r a n e - c e l lw a l l c o n t i n u u m i n p l a n t s .E m e r g i n g l i n k s r e v i s i t e d .P l a n t P h y s i o l 1 3 3 : 4 8 2 - . 1 9 1 B a n n i g a nA . W i e d e m e i e rA M D " W i l l i a m s o n R E . O v e r a l l R L , B a s k i n T I ( 2 0 0 6 ) C o n i c a l m i c r o t u bule arrays lose unilbrm alignment between cells and are or1'zalin resistant inthe Artthidopsts mutant, r'arllall,r'suollen 6. Plant Cell Physiol 41:919-958 B a r t o l o M E , C a r t e r J V ( l 9 9 l a ) M i c r o t u b u l e s i n t h e m e s o p h y l l c e l l s o f n o n a c c l i m a t e da n d c o l d a c c l i m a t e ds p i n a c h .P l a n t P h y s i o l 9 7 : l 7 - 5 - 1 8I B a r t o l o M E , C a r t e r J V ( l 9 9 l b ) E f f e c t o f m i c r o t u b u l e s t a b i l i z a t i o no n t h e f r e e z i n g t o l e r a n c eo f m e s o p h y l lc e l l s o f s p i n a c h . P l a n t P h y s i o l 9 7 : 1 8 2 - 1 8 7 Bartolo ME. Carter JV (1992) Lithium clecreasescold-induced rnicrotubule depolymerizarion in m e s o p h y l l c e l l s o f s p i n a c h .P l a n t P h y s i o l 9 9 1 11 6 - 1 1 1 8 B a s k i n T l ( 2 0 0 | ) O n t h e a l i g n m e n to f c e l l u l o s em i c r o l i b r i l sb y c o r t i c a l m i c r o t u b u l e s :a r e v i e w a n d a m o d e l . P r o t o p l a s m a2 l - 5 :l - 5 0 - 1 7I Baskin TI. Bivens NJ (19951 Stimulation of radial expansion inArabidopsis roots by rnhibitors ol actornyosinand vesicle secretionbut not by various inhibitors of metabolism. Planta I 97:-5l;l-5 2 I Baskin Tl. Wilson JE ( 1997) lnhibitors of protein kinases and phosphalasesalter root morpholo-u1' and disor,sanizecortical microtubules. Plant Physiol I l3:493-502 Bichet A, Desnos T, Turner S. Grandjean O, Höfte H (2001) BOTEROI is required fbr normal orientation rrf cortical microtubules and anisolropic cell expansion in Arubidopsis. Pl,tnt J 25:137-148 B i s g r o v e S R . L e e Y R J , L i u B , P e t e r sN T . K r o p f D L ( 2 0 0 8 ) T h e r n i c r o r u b u l ep l u s - e n d b i n d i n g protein EB I functions in root responsesto touch and eravitl' signals in Arabidopsis. Plant Cell 20:396--zlI0 B j ö r k m a n T ( 1 9 3 8 ) P e r c e p t i o no f g r a v i t y b y p l a n t s .A d v B o t R e s l 5 : l - . 1 Blancaflor EB (2000) Cortical actin filaments potentially interact with cortical microtubules in regulating polarity of cell expansion in primarv roots of mtize (Zea rtar,.sL.). J Plant Crowth Regul t9:.106--,114 B l a n c a f l o r E B . H a s e n s t e i nK H ( 1 9 9 3 ) O r g a n i z a t i o no f c o r t i c a l m i c r o t u b u l e si n g r a v i r e s p o n d i n r m a i z e r o o t s .P l a n t a l 9 l : 2 3 0 - 2 3 7 Bokros CL, Hu-edahlJD, Blumenthal SSD, Morejohn LC (1996) Proteolltic analysis of polyrrerized maize tubulin: regulation of microtubule stability to low temperature and Car* bv the c a r b o x y l t e m i n u s o f B - t u b u l i n .P l a n t C e l l E n v i r o n l 9 : 5 3 9 - - 5 : 1 8 B r a a m J , D a v i s R W ( 1 9 9 0 ) R a i n - . w i n d - , a n d t o u c h - i n d u c e de x p r e s s i o no f c a l m o d u l i n a n d c a l m o d u l i n - r e i a t e dg e n e si n A r a b i d o p s i s .C e l l 6 0 : 3 5 7 - 3 6 , 1 B r e v i a r i o D ( 2 0 0 8 ) P l a n t t u b u l i n g e n e s :r e g u l a t o r ya n d e v o l u l i o n a r y a s p e c t s .P l a n t C e l l M o n o g r ll:207 232 B r o w n R C . L e m m o n B E ( 2 0 0 7 ) T h e p l e i o m o r p h i c p l a n t M T O C : A n e v o l u t i o n a r - vp e r r p t c r i v e . J I n t P l a n t B i o l , 1 9 : l 1 , 1 2 - ll - 5 3 Buder J ( I 920) Neue phototropische Fundamenralversuche.Ber Dtsch Bot Ges 3ti: I 0-l 9 B u d e r J ( 1 9 6 1 ) D e r G e o t r o p i s m u sd e r C h a r a c e e n r h i z o i c l e B.e r D t s c h B o t G e s 1 4 : 1 1 L 3 B u r k D H . Y e Z H ( 2 0 0 2 ) A l t e r a t i o n o f o r i e n t e dd e p o s i t i o no f c e l l u l o s em i c r o t i b r i l s b y n r u t a t i o no f a k a t a n i n - l i k em i c r o t u b u l e s e v e r i n sn r o t e i n . P l a n t C e l l 1 . 1 : 2 1 4 5 - 2 1 6 0 P. Nick ti4 Burk DH, Liu B, Zhong R. Morrison WH, Ye ZH (2001) A katanin-like protein regulates normal cell wall biosynthesis and cell elongation. Plant Cell l3:807-827 Buschmann H, Fabri CO, Hauptmann M, Hutzler P, Laux T, Lloyd CW, Schäffner AR (2004) Helical growth of the Arabidopsis mutant tortifolial reveals a plant-specific microtubulea s s o c i a t e dp r o t e i n .C u n B i o l l 4 : 1 5 1 5 - 1 5 2 1 Camilleri C. Azimzadeh J, Pastuglia M, Bellini C, Grandjean O, Bouchez D (2002) The Arabidopsis TONNEAIJ2 gene encodes a putative novel PP2A regulatory subunit essential for the control of cortical cytoskeleton. Plant Cell l4:833-845 Campanoni P, Blasius B, Nick P (2003) Auxin transport synchronizesthe pattem of cell division in a t o b a c c oc e l l l i n e . P l a n t P h y s i o l 1 3 3 : 1 2 5 1 - 1 2 6 0 Canut H, Carrasco A, Galaud J-P, CassanC, Bouyssou H, Vita N, Ferrara P, Pont-Lezica R ( 1998) High affinity RGD-binding sites at the plasma membrane of Arabidopsis thaliana links the cell wall. Plant J l6:63-71 Cdrdenas L, Lovy-Wheeler A, Kunkel JG, Hepler PK (2008) Pollen tube growth oscillations and intracellular calcium levels are reversibly modulated by actin polymerization. Plant Physiol 146:l6ll-1621 Chan J, Calder G, Fox S, Lloyd C (2007) Cortical microtubule arrays undergo rotary movements in Arabidopsis hypocotyl epidermal cells. Nat Cell Biol 9:l7l-175 Collings DA (2008) Crossed-wires: interactions and cross-talk between the microtubule and micro. lament networks in plants. Plant Cell Monogr ll:4'7-'79 Collings DA, Lill AW, Himmelspach R, Wasteneys GO (2006) Hypersensitivily to cytoskeletal antagonistsdemonstrates microtubule-microfilament cross-talk in the control of root elongaIionin Arabidopsis thaliana. New Phytol 110:275-290 Dhonukshe P, Mathur J, Hülskamp M, Gadella TWJ (2005) Microtubule plus-endsreveal essential links between intracellular polarization and localized modulation of endocytosis during division-plane establishment in plant cells. BMC Biol 3:l l Ding JP, Pickard BG (1993) Mechanosensory calcium-selective cation channels in epidermal cells.PlantJ 3:83-l l0 Durso NA, Cyr RJ (1994) A calmodulin-sensitive interaction between microtubules and a higher plant homolog of elongation factor lcr. Plant Cell 6:893-905 Eckert BS, Yeagle PL (1988) Acrylamide treatment of PtKl cells causes dephosphorylation of keratin polypeptides. Cell Motil Cytoskelet ll:24-30 Edwards ES, Roux SJ (1994) Limited period of graviresponsivenessin germinating spores of C eratopteris richurdii. Planta 195: I 5G-152 Edwards ES, Roux SJ (1997) The influence of gravity and light on developmental polarity of single cells of Ceratopteris richardii gametophytes.Biol Bull 192:139-140 Elinson RP, Rowning B (1988) Transient array of parallel microtubules in frog eggs: potential tracks for a cytoplasmic rotation that specilies the dorso-ventral axis. Dev Biol 128:185-197 Fisher DD, Cyr RJ (1993) Calcium levels affect the ability to immunolocalize calmodulin to cortical microtubules. Plant Physiol l0:543-55 I Frey N, Klotz J, Nick P (2009) Dynamic bridges - a calponin-domain kinesin from rice links actin filaments and microtubules in both cycling and non-cycling cells. Plant Cell Physiol 50: 1493-1506 Frey N, Klotz J, Nick P (2010) A kinesin with calponin-homology domain is involved in premitotic nuclear migration. J Exp Bot 6l:3423-3431 Funada R (2008) Microtubules and the control of wood formation. Plant Cell Monogr I l:83-l l9 Furutani I, Watanabe Y, Prieto R, Masukawa M, Suzuki K, Naoi K, Thitamadee S, Shikanai T, Hashimoto T (2000) The SPIRAL genes are required for directional control of cell plates elongation in Arahidop sis t hali ana. Development 121:44434453 Gardiner JC, Harper JDI, Weerakoon ND, Collings DA, Ritchie S, Gilroy S, Cyr RJ, Marc J (2001) A 90-kD phospholipaseD fiom tobacco binds to microtubules and the plasma membrane. Plant Cell 13:2143-2158 P. Nick '..: .:: iri.t'protein r e g u l a t e sn o r m a l - .t. , , r J ( \ \ ' . S c h ä f T n e rA R ( 2 0 0 4 ) . . . . . r p l u n t - s p e c i f i cm i c r o t u b u l e - !,P-:. , t B'rruehezD (2002) The Arabir : . . . . 1 1 ( ) r s\ u b u n i t e S s e n t i afl O r t h e ' r. - the l l r t t e m o f c e l l d i v i s i o ni n li 1, . \ Fr.rrirrr P. Pont-Lezica R (1998) -ti 1 .;irr,lrrlrsi.r thaliana links the cell _ t . . , i n t u b e g r o w t h o s c i l l a t i o n sa n d - : r : r I ) o l \ m e r i z a t i o n .P l a n t P h y s i o l I_-- : r . \ . u n d c r g or o t a r y m o v e m e n t si n r-5 ,.r betueen the microtubule and :, ,- ' '.:-: . i f \: ,:.-i.; Hi perrensitivity to cytoskeletal :.,,r in the control of root elonga: , t u l . u l ep l u s - e n d sr e v e a le s s e n t i a l '.iulation of endocytosisduring .L .lrtion channelsin epidermal !:, :.\ r-c.nmicrotubules and a higher . :-:, J . L \ ! . i l u s e sd e p h o s p h o r y l a t i o no f i, .rrrr'\\ in germinating spores of f, :^: :rr ,:r . 'i rrn dsv.l.tmental polarity of I.rl: I 39- I 40 :,:,rbules in fiog eggs:potential : . : . . i \ 1 \ .D e v B i o l 1 2 8 : 1 8 5 - 1 9 7 : : r r n u n o l o c a l i z ec a l m o d u l i n t o . , r ' k r r r e . i nI ' r o mr i c e l i n k sa c t i n - -cil'. Plant Cetl Physiol 50: ,.t)r\ domain is involved in l : - l _ ::.j :i.,: ? t l ' . . i n tC e l l M o n o g r I l : 8 3 - l l 9 i - . T h i t a r n a d e eS , S h i k a n a i T , ',:r,'nal control of cell plates a i : :,'r S.Cyr RJ.MarcJ (2001) .r :irt piasmamembrane. Plant Mechanics of the Cytoskeleton 8-5 GeigerB, BershadskyA (2001)Assemblyand mechanosensory functionof focal contacrs.Curr Opin Cell Biol l3:584-592 GensJS, Fujiki M. PickardBG (2000)Arabinogalactan proteinand wall-associated kinasein a plasmalemmal reticulumwith specialized vertices.Protoplasma 212:l l5-134 GerhartJ, UbbelesG, Black S, Hara K, KirschnerM (1981)A reinvestigation of the role of the grey crescentin axis formationin Xenopus/aells. Nature292:5ll-516 GianconiFG, RuoslahtiE (1999)Integrinsignaling. Science285:1028-1032 GianfS, Qin X, FaoroF, BreviarioD ( 1998)In rice,oryzalinandabscisicaciddiffbrentiallyaffect tubulin mRNA and protein levels. Planta205:334-341 GiddingsTH, StaehelinA (1988)Spatialrelationshipbetweenmicrotubulesandplasmamembrane rosettesduring the depositionof primary wall microfibrilsin Closterium.rper'.Planta 173: 22-30 GiddingsTH, StaehelinA (1991) Microtubule-mediated control of microllbril deposition.A re-examination of the hypothesis.In: Lloyd CW (ed) The cytoskeletalbasisof plant growth and form. Academic,London,pp 85-99 GiererA (1981)Generationof biologicalpattemsand form: somephysical,mathematical, and logicalaspects. ProgrBiophysMol Biol 31:141 Gittes F, Mickey B, NettletonJ, Howard J (1993) Flexual rigidity of microtubulesand acrin filarnentsmeasuredfrom thermal fluctuationsin shape.J Cell Biol 120:923-934 CodboleR. MichalkeW. Nick P. HertelR (2000)Cyroikeletal drugsandgravity-induced lareral auxin transportin rice coleoptiles.PlantBiol 2:176-l8l GoebelK (1908)Einleitungin die experimentelle Morphologieder Pflanzen.Teubner,Leipzig, pp 218-251 GoodeBL, Drubin DG, BamesG (2000)Functionalcooperationbetweenthe microtubuleand actincytoskeletons. Curr Opin Cell Biol l2:63-7 I GossotO, GeitmannA (2007)Pollentubegrowth:copingwith mechanicalobsracles involvesthe cytoskeleton.Planta226:4O54 | 6 GrabskiS, SchindlerM ( 1996)Auxins andcytokininsasantipodalmodulatorsof elasticitywithin the actinnetworkof plantcells.PlantPhysiolI 10:965-9'70 GrabskiS, Amoys E, BuschB, SchindlerM (1998)Regulationof actin tensionin plant cells by kinasesand phosphatases. PlantPhysiol l16l2'79-290 GreenPB (1962)Mechanismfbr plantcellularmorphogenesis. Science138:140'1-1405 - a biophysicalview. Annu Rev Plantphysiol 3 l:51-82 GreenPB (1980)Organogenesis Gus-Mayer s, Naton B. Hahlbrock K, SchmelzerB (1998) Local mechanicalstimulation inducescomponentsof the pathogendefenseresponsein parsley.Proc Natl Acad Sci USA 95:8398-8403 GustinMC, SachsF, SigurdsonwJ, RuknudinA, Bowmanc ( | 991) Technicalcomments.Single channelmechanosensitive currents.Science253:1195-119'7 GutjahrC, Nick P (2006)Acrylamideinhibits gravitropismand destroysmicrotubulesin rice coleoptiles.Protoplasma 227:21| -222 HaberlandG ( 1900)Uberdie Perzeption desgeotropischen Reizes.Ber DtschBor Ges 18.261-212 HamadaT (200'7)Microtubule-associared proreinsin higherplants.J plant Res 120:79-98 HamantO, HeislerMG, JönssonH, Krupinski P, UyttewaalM, Bokov P, CorsonF, Sahlinp. BoudaoudA, Meyerowitz EM, Couder Y, Traas J (2008) Developmentalpatterningby mechanicalsignalsin Arabidopsis.Science322:1650-1655 HardhamAR, GreenPB, Lang JM (1980)Reorganization of corticalmicrotubulesand cellulose depositionduringleaf formalionof Graptopetalunt paraguuy-cnsc. Planta149:l8l-195 HasezawaS. Nozaki H (1999) Role of cortical microtubulesin the orientationof cellulose microfibrildepositionin higher-plantcells.Proroplasma 209:98-l04 HashimotoT, Kato T (2006)Conicalcontrolof plantmicrotubules. Curr Opin PtantBiol 9:5-l I HeathIB (19'/4)A unifiedhypothesisfor the role of membraneboundenzymecomplexesancl microtubulesin plantcell wall synthesis. J Theor Biol 48:445449 86 P. Nick H e p l e r P K . V i c l a l i L . C h e u n g A Y ( 2 0 0 1 ) P o l a r i z e dc e l l g r o w t h i n h i g h e r p l a n t s .A n n u R e v C e l l Dev Biol l7:l-59-187 Hertel R, Friedrich U ( 1973) Abhängigkcit der geotropischenKrümmun,q der Chura-Rhizoide von der Zentrifugalbeschleunigung.Z Pflanzenphysiol 70: I 73- I 84 H i m m e l s p a c hR . W y m e r C L . L l o y d C W . N i c k P ( I 9 9 9 ) G r a v i t y - i n d u c e dr e o r i e n t a t i o no f c o r t i c a l microtubules observed in vivo. Plant J l8:'{49-453 Himmelspach R. Nick P (2001) Gravitropic microtubule reorientation can be uncoupled frorn g r o w t h . P l a n t a 2 l 2 : 1 8 , 1 - l8 9 Hodick D (199,1)Negative gravitropisrn inChara protonemata: a model integrating the opposite eravitropic responsesof protonemata and rhizoids. Planta I 95:'13-49 H o l u b o w i c z T " B o e A A ( 1 9 6 9 ) D e v e l o p m e n t o f c o l d h a r d i n e s si n a p p l e s e e d l i n g st r e a t e d w i t h , s i b b c r e l l i ca c i d a n d a b s c i s i ca c i d . J A m S o c H o r l i c S c i 9 ' 1 : 6 6 1 - 6 6 4 H o l w e g C . S ü l j l i n C . N i c k P ( 2 0 0 4 ) C a p t u r i n g i n - v i v o d v n a m i c s o l t h e a c t i n c y t o s k e l e t o n .P l a n t C e 1 1P h y s i o l 4 5 : 8 5 5 - 8 6 3 H u s h J M , H a w e s C R , O v e r a l l R L ( 1 9 9 0 )I n t e r p h a s em i c r o t u b u l er e - o r i e n t a t i o np r e d i c t sa n e w c e l l polarity in wounded pea roots. J Cell Sci 96:47-61 I g a r a s h iH . O r i i H , M o r i H . S h i m m e n T . S o n o b e S ( 2 0 0 0 ) I s o l a t i o n o f a n o v e l 1 9 0 k D a p r o t e i n fionr tobircco BY-2 cells: possible involvement in the interaction between irctin filaments and m i c r o t u b u l e s .P l a n t C e l l P h y s i o l 4 l : 9 2 ( I - 9 3 I lkushirna T. Shimmen T (200-5) Mechano-sensitive orientation of cortical microtubules during gravitropisnt in azuki bean epicotyls. J Plant Res I I 8:19-26 Ingber DE (2003a) Tensegrity l: cell structure;rnd hierarchicalsystemsbiology..l Cell Sci ll6:ll-57-1173 lngber DE (2003b) Tensegrity II: how structural networks influence cellular infbrmation process i n g n e t w o r k s .J C e l l S c i l l 6 : 1 3 9 7 - 1 4 0 t 3 lrving RM ( 1969) Characrerization and role of an endogenous inhibitor in the induction of cold Plant Physiol 'l'l:801-805 hardinessin r\cer neg,unchr. Plant Physiol 43:9- I 3 Irving RM, Lanphear FO ( 196i3)Regulationof cold hardinessin Acer ne,{,unrlo. J a c o b F ( 1 9 7 7 ) E v o l u t i o n a n d t i n k e r i n g . S c i e n c e1 9 6 : l l 6 l - l 1 6 6 Jaff'e MJ, Leopold AC, Staples RA (2002) Thigmo responses in plants and fungi. Am J Bot 89:375-382 Janmey PA. Weitz DA (2004) Dealing with mechanics: mechanisms of lbrce transduction in cells. TrendsBiochem Sci 29:364-370 J i a n L C . S u n L H , L i n Z P ( 1 9 8 9 ) S t u d i e so n m i c r o t u b u l e c o l d s t a b i l i t - vi n r e l a l i o n t o p l a n t c o l d h a r d i n e s s .A c t a B o t S i n 3 l : 7 3 7 - 7 4 1 JonesRS, Mitchell CA (19U9)Calcium ion involvement in growth rnhibition of mechanically stressedsoybean Gl.r'tine rln.r seedlings. Physiol Plant 76:59U-602 Kakirnoto T. Shibaoka H (1987) Actin filaments in the preprophase band and phragmoplast of t o b a c c oc e l l s . P r o t o p l a s m a1 4 0 :l 5 l - 1 5 6 K a r k i S , H o l z b a u r E L ( 1 9 9 9 ) C y t o p l a s m i cd y n e i n a n d d y n a c t i n i n c e l l d i v i s i o n a n d i n t r a c e l l u l a r transport. Curr Opin Cell Biol l:4-5-53 K a t s u t aJ . S h i b a o k aH ( I 9 8 8) T h e r o l e s o f t h e c y t o s k e l e t o na n d t h e c e l l w a l l i n n u c l e a rp o si t r o ni n g i n t o b a c c oB Y - 2 c e l l s . P l a n t C e l l P h y s i o l 2 9 : 4 0 3 - z l l 3 K e l l A . G l a s e r R W ( 1 9 9 3 ) O n t h e m e c h a n i c a la n d d y n a m i c p r o p e r t i e so 1 ' p l a n t - c e l lm e m b r a n e s : t h c i r r o l e i n g r o w t h . d i r e c t g e n e t r a n s f e ra n d p r o t o p l a s tf u s i o n . J T h e o r B i o l 1 6 0 : 4 1 - 6 2 Kennard JL, Cleary AL (1997) Pre-mitotic nuclear migration in subsidiarymother cells of 'fradest'antiu occurs in Gl of the cell cycle and requires F-actin. Cell Motil Cytoskelet 36:55-67 Ke-rr GP, Carter JV (1990) Relationship between fieezing tolerance of root-tip cells and cold stability of'microtubules in rye (Seta/e cereule L. Cv Purna). Plant Physiol 93:11-82 Kimura S. Laosinchai W. ltoh T, Cui X, Linder CR. Brown RM ( 1999) hnmuno,eold labeling of rosette terminal cellulose-synthesizingcomplexes in the vascular plant I'i.qna ungularis.Plant Cell I l:2075-2086 p. Nick r : r l r ' p l a n t s .A n n u R e v C e l l ' . . n 1J c r C / l a i z - R h i z o i d ev o n : . r !J r l r e { t r i e n t a t i o no f c o r t i c a l . . , 1 r , ' ne a r ) b e u n c o u p l e d f r o n t ' ' . i , r r r t c u r a t i ntgh c o p p o : i t e -: r- lr.l : . t P P l gs' e g c l l l n g st r e a t e d w i t h / r tr-i : ihc uetin cytoskeletonP . lant . , rr ! ' D t i l t i o np r e d i c t sa n e w c e l l l r t. : , ' t : r t ) ( ) \ r ' l1 9 0 k D e p r o t e i n : r h t t u e e n a c t i n l i l a m e n t sa n d ' -()ftic'rl m i c r o t u b u l e sd u r i n g . . . r ( r r r \h i o l o g l. J C c l l S c i ..' ir'llular inlbrmation procesr ' . l r ( r ri n t h e i n d u c t i o n o f c o l d , t r i / ? ( 1 .P) .l a n tP h y s i o l 4 3 : 9 - 1 3 : . l : u r l sa n d f u n g i . A m J B o t . . , l 1 ( ) r c at r a n s d u c t i o ni n c e l , . . . rrrr in relationto plant cold '' rrhibition of mechanically . . - l . . r n t la n d p h r a g m o p l a s to f - -. Jir r:ion and intracellular .. . .l.l l i n n u c l e a rp o s i t i o n i n g ' --,'l plunt-cell membranes: l: -',r Biol 160:41-62 ...^.r,liar) mother cells of -:, (c.ll \lotil Cytoskeler ' . r ' r , o t - t i pc e l l s a n d c o l d ' I ' r : r' i o l 9 3 : 7 1 - 8 2 ' lrrnrunosoldlabelingof ' . : i igrirr un,qularis.Plant Mechanics of the Cytoskeleton 87 K i s h i m o t o U ( 1 9 6 8 ) R e s p o n s eo l C h a r a i n t e m o d e s t o m e c h a n i c a l s t i n l u l a t i o n . A n n R e p B i o l Works Fac Sci OsakaUniv l6:61-66 Knight MR, Campbell AK. Smith SM, TrewavasAJ (199l)Transgenic planr aequorin rL-porrs the ef-fects of touch and cold-shock and elicitors on cytoplasmic calciunt. Nature 352:52,1-5.16 Kobayashi I. Kobayashi Y (2008) Microtubules and pathoeen def'ence. Planr Cell Monogr I l:l2l-140 Komis G, Apostolakos P. Galatis B (2002) Hyperosmotic stress induces fbrmation ol tubulin macrotubules in root-tip cells of fi'iticant turgitlunr. their probable involvement in protoplast v o l u m e c o r r t r o l .P l a n t C e l l P h y s i o l 4 3 : 9 1 l - 9 2 2 Komis G. Quader H. Galatis B, Apostolakos P (2006) Macrotubule-dependent protoplast volume regulation in plasmolysed root-tip cells of Triticunt turgidunt: involvement o1'phospholipaseD. N e w P h y t o l 1 1| : 1 3 1 1 5 0 Kune C (2005) A possible unifying principle fbr mechanosensation.Nature 136:641,651 KutscheraU (2008) The outer epidermal wall: desisn rnd physiological role of u cornporire s t r u c t u r eA. n n B o t l 0 l : 6 1 5 - 6 2 1 K u z n e t s o v O . A . H a s e n s t e i nK H ( 1 9 9 6 ) M a g n e t o p h o r e t i c i n d u c t i o n o f r o o t c u r v a t u r e . P l a n t a 198:87-9.{ L a w r e n c eC J . M o r r i s N R . M e a g h e r R B . D a w e R K ( 2 0 0 1 ) D y n e i n s h a v e r u n t h e i r c o u r s e i n p l a n t lineage. Tratfic 2:362-363 L e d b e t t e rM C , P o r t e rK R ( 1 9 6 3 )A m i c r o t u b u l ei n p l a n t c e l l l l n e s t m c t u r e .J C e l l B i o l l 2 : 2 3 9 - 2 5 0 L i n t i l h a c P M . V c s e c k y T B ( 1 9 8 4 ) S t r e s s - i n d u c e da l i g n m e n t o f d i v i s i o n p l a n e i n p l a n t t i s s u e s grown in vitro. Nature 307:363-364 Lloyd CW, Traas JA (1988) The role of F-actin in determining the division plane of carrot s u s p e n s i o nc e i l s . D r u g S t u d D e v 1 0 2 : 2 l l - 2 2 1 Los DA. Murata N (20021)Membrane fluidity and its roles in the perception of environmental s i g n a l s .B i o c h i m B i o p h y s A c t a 1 6 6 6 : l z l 2 - 1 5 7 Lucas J, Shaw SL (200ti) Cortical microtubule arrays in the Arahitlopsis seedling. Curr Opin Plant Biol I l:9zl- 9f.i Lyons JM (1973) Chilling injury in plants. Annu Rev Plant Physiol 24:421-5-'166 M a i s c h J . N i c k P ( 2 0 0 7 ) A c t i n i s i n v o l v e d i n a u x i n - d e p e n d e npt a t t e r n i n g .P l a n t P h v s i o l . . P l a n t Physiol l.iJ: 1695-170.+. M c C l i n t o n R S , S u n g Z R ( 1 9 9 ' 7 )O r g a n i z a t i o no f c o r t i c a l m i c r o t u b u l e sa t t h e p l a s m a m e m b r a n ei n A r u b i d o p s i t P l a n t a 2 0 1: 2 5 2 - 2 6 0 M o d i g C , S t r ö m b e r gE . W a l l i n M ( 1 9 9 4 ) D i t t e r e n t s t a b i l i t y o f p o s t t r a n s l a t i o n a l l ym o d i l i e d b r a i n m i c r o t u b u l e si s o l a t e dl i o m c o l d - t e m p e r a t ef i s h . M o l C e l l B i o c h e m 1 3 0 : 1 3 7 1 , 1 7 M o n r o y A F , S a r h a nF , D h i n d s a R S ( 1 9 9 3 ) C o l d - i n d u c e dc h a n g e si n f r e e z i n g t o l e r a n c e .p r o t e i n phosphorylation, and gene expression. Plant Physiol 102:1221-1235 M o r r i s N R ( 2 ( X ) 3 )N u c l e a r p o s i t i o n i n , et:h e m e a n s i s a t t h e e n d s .C u r r O p i n C e l l B i o l l 5 : 5 : 1 - - 5 9 Moseley JB. Bartolini F, Okada K. Wen Y. Cundersen GG, Goode BL (2007) Regulated binding o f a d e n o m a t o u sp o l y p o s i sc o l i p r o t e i n t o a c t i n . J B i o l C h e r n 2 8 2 : 1 2 6 6 1 1 2 6 6 8 Mulder B, Schell J, Emons AM (20021)How the geometrical model for plant cell wall lbnnation e n a b l e st h e p r o d u c t i o no f a r a n d o n t e x t u r e .C e l l u l o s e I l : 3 9 5 - 4 0 1 Murata T, Wada M ( l99l) Efl'ects of centritugation on preprophase-bandfbrrnation in Atliuntunt protonemata. Planta I Il3:39 l-398 M u r a t a N , l s h i z a k i - N i s h i z a w aO , H i g a s h i H . T a s a k a Y . N i s h i d a I ( 1 9 9 2 ) G e n e t i c a l l ye n g i n e e r e d a l t e r a t i o ni n c h i l l i n g s e n s i t i v i t yo f p l a n t s .N a t u r e 3 5 6 : 7l 0 - 7 I 3 Nemec B (1900) Uber die Art der Wahrnehmung des Schwerkrafireizes bei den Pflanzen. Ber Dtsch Boi ües l8:241 24-5 N i c k P ( 2 0 0 8 a )C o n t r o l o 1 ' c e l l a x i s . P l a n t C e l l M o n o g r I l : 3 - 4 6 N i c k P ( 2 0 0 8 h ) N { i c r o t u b u l e sa s s e n s o r sf o r a b i o t i c s t i r n u l i .P l a n t C e l l M o n o g r I l : 1 7 - 5 - 2 0 3 Nick P, Furuya M ( 1996) Buder revisited - cell and organ polarity during phototropism. Plant Cell Environl9:l179-l ll37 88 P. Nick Nick P. Schäfer E, Hertel R, Furuya M ( l99l ) On the putative role of microtubules in gravitropism of maize coleoptiles. Plant Cell Physiol 32:873-880 Nick P, Yatou O, Furuya M, Lambert AM (1994) Auxin-dependent microtubule responsesand seedling development are affected in a rice mutant resistant to EPC. Plant J 6:651-663 Nick P, Godbol6 R, Wang QY (1997) Probing rice gravitropism with cytoskeletal drugs and cytoskeletal mutants. Biol Bull 192:141-143 Nick P. Han M, An G (2009) Auxin stimulates its own transport by actin reorganization. Plant P h y s i o ll 5 l : 1 5 5 - 1 6 7 Niklas KJ (1992) Plant biomechanics. An engineering approach to plant form and function. University of Chicago Press, Chicago On AW, Helmke BP, Blackman BR, Schwartz MA (2006) Mechanisms of mechanotransduction. Dev Cell l0:l l-20 Panteris E (2008) Cortical actin filaments at the division site ofmitotic plant cells: a reconsidera'actin-depleted zone'. New Phytol 179:334-341 tion of the CR, Ehrhardt DW (2006) Visualization of cellulose synthase demonAR. Somerville Paredez stratesfunctional association with microtubules. Science 3 l2:1491-1495 Parthasarathy MV. Perdue TD, Witztum A, Alvemaz J (1985) Actin network as a normal '72:1318-1323 component of the cytoskeleton in many vascular plant cells. Am J Bot Pickard BG (2008) "second extrinsic organizational mechanism" for orienting cellulose: modeling a role for the plasmalemmal reticulum. Protoplasma 233:1*29 Pickard BG, Fujiki M (2005) Ca'* pulsation in BY-2 cells and evidence fbr control of mechanosensoryCa2*-selectivechannels by the plasmalemmal reticulum. Funct Plant Biol 32:863-879 Pihakaski-MaunsbachK. Puhakainen T ( | 995) Effect of cold exposure on cortical microtubules of rye (.Secalecereale) as observed by immunocytochemistry' Physiol Plant 93:563--571 Preston RD (1988) Cellulose-microfibril-orienting mechanisms in plant cell walls. Planta l'74:61-74 Preuss ML, Kovar DR, Lee YR, Staiger CJ, Delmer DP, Liu B (2004) A planrspecific kinesin binds to actin microlilaments and interacts with cortical microtubules in cotton fibers. Plant Physiol 136:3945-3955 Rawitscher F (1932) Der Geotropismus der Pflanzen. Fischer. Jena Richardson D, Simmons M, Reddy A (2006) Comprehensive comparative analysis of kinesins in photosynthetic eukaryotes. BMC Genomics 7:18 Rikin A, Richmond AE (1976) Amelioration of chilling injuries in cucumber seedlingsby abscisic acid. Physiol Plant 38:95-97 Rikin A, Waldman M, Richmond AE, Dovrat A (197-5)Hormonal regulation of morphogenesis and cold resistance.I. Modilications by abscisic acid and gibberellic acid in alfalfa (Medicago s a t i v a L . ) s e e d l i n g s J. E x p B o t 2 6 : 1 7 5 - 1 8 3 Rikin A, Arsmon D, Gitler C (1980) Chilling injury in cotton (Gos.r,)'piumhirsutum L.): effects of antimicrotubular drugs. Plant Cell Physiol 2l:829-837 Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD (2001) Focal contacts as mechanosensors:extemally applied local mechanical force induces growth of focal contacts by an mDial-dependent and ROCK-independent m e c h a n i s m .J C e l l B i o l 1 5 3 : l 1 7 5 - l 1 8 5 Robby T (1996) A new architecture. Yale Academic Press,New Haven Rodriguez OC, Schaef'erAW, Mandato CA, Forscher P, Bement WM, Waterman-Storer CM (2003) Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat Cell Biol 5:599-609 Sachs J ( 1880) Stoff und Form der Pflanzenorgane.Arb Bot Inst Würzburg 2:469419 Sachs F, Molis CE (1998) Mechanosensitive ion channels in nonspecialized cells. Rev Physiol Biochem Pharmacol 132:l-7'7 Sainsbury F, Collings DA, Mackun K, Gardiner J, Harper JDI, Marc J (2008) Developmental reorientation oftransverse cortical microtubules to longitudinal directions: a role f'or actomyosinbased streaming and partial microtubule-membrane detachment. Plant J 56:l l6-13l P. Nick :1:: . . ' , . , t n t r c r o t u b u l e si n g r a v i t r o p i s m r . " r - : ' . - : . j i r ) t n r i c r o t u b u l er e s p o n s e sa n d i1. . .: [ : I ' ] ( ] .P l a n t J 6 : 6 5 1 - 6 6 3 - : .::r rrirh cytoskeletal drugs and a-.. - :-., .: ,:: r\ actin reorganization. Plant :: :::" . . ...r t!) plant tbrm and function. \ 1 - . .: t . r n r \ n t \o f m e c h a n o t r a n s d u c t i o n . r . 1. r.-j t i t l ( ) l l ep l a n t c e l l s : a r e c o n s i d e r a - . . . : ; ; ; r r i c e l l u l o s e s y n t h a s ed e m o n - ..\i :- .{ctin network as a normal r-r -. . \rl J Llot12:1318-1323 r - - ' - : . i ' r l o r s 1 i a n , i n t c e l l u l o s e :m o d e l !-- -.-ii - 19 - r .. .: .: - r rdence fbr control of mechan: : ' : . - . . . . . n rF u n c t P l a n t B i o l 3 2 : 8 6 3 - 8 1 9 r : , :.,',urc r)n cortical microtubules of , ' - . . - . l ' i r r . r o l P l a n t 9 3 : 5 6 3 ^ 5 ' lI ri, - :r ..:r.\ in plant cell walls. Planta )P - I J l ( i O + ) A p l a n t - s p e c i f i ck i n e s i n < 1 : , . , : - r , r l u b u l e si n c o t t o n f i b e r s . P l a n t : : i l r i t \ e a n a l y s i so f k i n e s i n si n . . u r r t h c r e e d l i n g sb y a b s c i s i c , ^ :1 I r-,: - . , t : t u r tl t i r s u t u mL . ) : e f f e c t so f .:_ F r - . b. ?.. . , . r ( ! u l i . l t i o no f m o r p h o g e n e s i s ^-:rllri acid in alfalfa (Medicago I \- t :. :i' \.:i-nl\it S. Kam Z, Geiger B, ' - . ; : r . ,t tr . r p p l i e d local mechanical ., ..i:nr .rnd ROCK-independent :t.r\ill . . : \ \ \ 1 . W a t e r m a n - S t o r eCr M - . - . : t : r n d m o r p h o g e n e s i sN . at ' 3 . r€ "' -:/adr-! ):169419 . : - - ' -r . r l i z . ' dc e l l s . R e v P h y s i o l E-.8' \1.,:- ,l l(X)13.y Developmental I :---:.,lt. arolctbraCtomyOSin: ' . , ; : lJ 5 6 : l l 6 - 1 3 l l.-i:Jt " : r i-. Mechanics of the Cvtoskeleton 89 SakiyamaM, ShibaokaH (1990)Effectsof abscisicacid on the orientationand cold stabilityof corticalmicrotubulesin epicotylcells of the dwarf pea.hotoplasma 157:165-l'71 SamuelsAL, GiddingsTH, StaehelinLA (1995)Cytokinesisin tobaccoBY-2 an<lroot tip cellsa new modelof cell plateformationin higherplanrs.J Cell Biol 130:1345-1357 SandbladL, BuschKE, TittmannP, GrossH. BrunnerD, HoengerA (2006)The SchizosatchuromtcespombeEBI homologMal3p binds and stabilizesthe microtubulelatticeseam.Cell 121:1415*1424 SangwanV, FouldsI, Singh J, DhindsaRS (2001) Cold-activationof Brassicanapus BNtl-5 promoteris mediatedby structuralchangesin membranes andcytoskeleton, andrequiresCa2t influx.PlantJ 2'7:l-12 SanoT, Higaki r, oda Y, Hayashir, Hasezawa s (2005)Appearance of actinmicrofilamenr'twin peaks'in mitosisand their functionin cell platetbmation, as imagedin tobaccoBY-2 cells expressing GFP-fimbrin.PlantJ 44:595-605 SatoY, KadotaA, WadaM ( 1999)MechanicallyInducedAvoidanceResponse of Chloroplasts in Fem ProtonemalCells.PlantPhysiol l2l:37-44 SchmelzerE (2002) Cell polarization,a crucial processin fungal defence.Trends Plant Sci 7:4ll4l5 SchmitAC, Nick P (2008)Microtubulesandthe evolutionof mitosis.PlantCell Monogr I I : 1500 233-266 SchwuchowJ, SackFD, HartmannE (1990)Microtubuledisruptionin gravitropicprotonemara of the mossCeratodon.Protoplasma159:6G-69 SeagullR (1990)The effectsofmicrotubuleandmicrofilamentdisruptingagentson cytoskeletal arraysand wall depositionin developingcottonfibers.Protoplasma159:44-59 SedbrookJC, Kaloriti D (2008)Microtubules,MAPs andplantdirectionalcell expansion. Trends PlantSci l3:303-310 ShibaokaT (1966)Action potenrialsin plant organs.Symp SocExp Biol 20:165-1t34 SieversA, SchröterK (1971)VersucheinerKausalanalyse der geotropischen Reakrionskette im C hara-P.hizoidPlanta96:339-353 SonobeS, ShibaokaH (1989)Cortical fine actin lilamentsin higher plant cells visualizedby rhodamine-phalloidin afterpretreatment with m-maleimidobenzoyl-N-hydrosuccinimide ester. Protoplasma148:8G86 TabonyJ, GladeN, Papaseit C, DemongeotJ (2004)Microtubuleself-organization asan example of the development of orderin living systems.J Biol PhysChem4:50-63 TakemotoD, HardhamAR (2004)The cytoskeleton asa regulatorandtargetofbiotic interactions in plants.PlantPhysiol136:3864-3876 TamuraK, NakataniK, MitsuiH, OhashiY, Takahashi H (1999)Characrerizarion of karD,a kinesinlike proteingenespecilicallyexpressed in floral tissuesof Arabidopsisthaliana. Gene230:23-32 Taylor DP, LeopoldAC ( 1992)Offsetof gravitropismin maizerootsby low temperarure. ASGSB Bull 6:75 TelewskiFW (2006)A unifiedhypothesis ofmechanoperception in plants.Am J Bot 93:1466_1476 ThimannKV, ReeseK, NachmikasVT ( 1992)Actin andtheelongationof plantcells.Protoplasma l 7 l :l 5 l - 1 6 6 Thitamadee S, TuchiharaK, HashimotoT (2002)Microtubulebasisfbr lefrhandedhelicalgrowth in Arahidopsis.Nature4 I 7: 193-196 ThomasDDS, Dunn DM, SeagullRW (1977)Rapidcytoplasmicresponses of oar coleoptilesto cytochalasin B, auxin,andcolchicine. CanJ Bot 55:1797-1800 ThompsonDW (1959)On growthandform. CambridgeUniversityPress,Cambridge,pp 465-6,14 Timauer JS, Bierer BE (2000)EBI proteinsregulatemicrotubuledynamics,cell polarity, and chromosomestability.J Cell Biol 149:161-766 ToriyamaH, JaffeMJ (1972)Migrationof calciumand its role in the regulationof seismonasry in the motor cell of Mimosaputlicu D. Plant Physiol 49:12-81 TraasJ, Bellini c, NacryP, Kronenberger J, BouchezD, CabocheM ( 1995)Normaldifferentiation pattemsin plantslackingmicrotubularpreprophase bands.Nature37-5:676-677 90 P. Nick T s v e t k o v A S , S a m s o n o vA , A k h m a n o v a A . G a l j a r t N . p o p o v s v ( 2 0 0 7 ) Microrubule-bindins p r o t e i n sC L A S P I a n d C L A S P 2 i n t e r a c tu , i t h a c t i n f i l a m e n t s .C e l l M o t i l C y r o s k e l e t6 ; 1 : -l59 - 5 3 0 Turing AM ( 19-52)The chemical basis ol morphogenesis.Philos Trans R Soc Lond SerB 23j:31-j2 Vantard M. Leviliiers N. Hill AM. Adoutte A. Lambert AM (1990) Incorporation c>fpurunteL.iur.tt a x o n e m a lt u b u l i n i n t o h i g h e r p l a n t c e l l s r e v e a l sf u n c t i o n a is i r e so f r n i . l r o t u b u l e a s s e m b l y .p r o c Natl Acad Sci USA 87:8825-1i829 v i t h a s . F r o e h l i c h J E . K o k s h a r o v ao . p y k e K A . v a n E r p H . o s t e r y o u n g KW (2003)ARC6 is a J-domain plastid division protein and an evolutionarv descendint oi the cyanobacterial cell d i v i s i o np r o t e i nF t n 2 . p l a n t C e l l l 5 : l 9 l t i _ 1 9 3 3 V ö c h t i n g H ( 18 7 8 ) Ü b e r O r e a n b i l d u n gi r r p f l a n z e n r e i c h C . o h e n .B o n n Vogelmann TC. BasselAR' Miller JH ( 198I ) Efl-ectsol microtubule-inhibitors on nuclear migrarion and rhizoid formation in genninating 1'ernspores(Onocleu .sensibilrs). protoplasma 109:295-3l6 voigt B, Tirnmers ACJ. Samal J. Müller J. Baluika F, Menzel D (200-5) bFP-FABD2 fusion construct allows lri lilo visualization oi the dynamic actin cvtoskeleton in all cells of Arabidopsis seedlings. Eur J Cell Biol li,1:59-5_60g Walker LM. Sack FD (19901 Amyloplasts as possible statoliths in gravitropic proronemaraof the ntoss Ceratotlon purplrreus. planta l8 I :71_77 W a l l e r F . N i c k P ( 1 9 9 7 ) R e s p o n s eo f a c t i n r n i c r o t i l a m e n t sd u r i n g p h y t o c h r o m e - c o n r r o l l e d growth of maize seedlinss. Protoplasma 200: I -5.1-162 Waller F, Riemann M. Nick P (2002) A role 1br actin-driven secretion in auxin-ipduceclgrowth. P r o t o p l a s m a2 1 9 7 2 - S l Wang QY. Nick P (199u) The auxin response of actin is altered in the rice mutant yitl-yang. Protoplasma 204:22-33 W a n g Q Y . N i c k P ( 2 0 0 1 ) C o l d a c c l i m a t i o n c a n i n c l u c em i c r o r u b u l a r cold srability in a manner distinct fiom abscisic acid. plant Cell phirsiol 42:()99-1005 wang YS. Motes CM. Mohamalawari DR. Blancaflor EB (2004) Green fluorescentprotein flsions tt:tArahidopsis fimbrin I fbr spatio-temporal irnaging of F-actin dynamics in roots. Cell Motil Cytoskeler 59:79-93 wang X' zhua L. Liu B. wang c, Jin L. Zhao e. yuan M (2007) Arabidopsis microtubureassociatedprotein I 8 functions in directional cell growth by destabilizing cor.ticalmicrotubules. Plant Cell l9:877-8t39 W a s t e n e y sG O ( 2 0 0 4 ) P r o s r e s si n u n d e r s t a n d i n gt h e r o l e o f m i c r o t u b u l e s i n p l a n t c e l l s .C u m o p i n Plant Biol 7:651-660 wasteneys Go. Galway ME (2003) Remodeling rhe cytoskereron fbr growth and fbrm: an overview with somenew views. Annu Rev planr Biol 54:691 122 w h i t t i n g t o n A T , v u g r e k o . w e i K J , H a s e n b e i nN c . S u g i m o t oK . R a s h b r o o k e M C . w a s r e n e y sc o ( 2 0 0 1 ) M O R I i s e s s e n t i a l f b r o r g a n i z i n g c o r t i cm i r il c r o r u b u l e s i n p l a n r s . N a r u r e 4 l l : 6 1 0 - 6 1 3 Wiesler B' Wang QY, Nick P (2002) The stability of cortical microtubules depends on their o n e n t a r i o n .P l a n t J 3 2 : 1 0 2 3 1 0 3 2 wymer C. Wymer SA' Cosgrove DJ. Cyr RJ (1996) Plant cell srowrh respondsto extemal fbrces and the responserequires intact microtubules. plant physiol I l0:u12.5_430 Xu T. Qu z'Yang X, Qin x. Xiong J, wang y, Ren D. Liu G (2009) A couon kinesinGhKCH2 l n t e r a c t sw i t h b o t h m i c r o t u b u l e sa n d m i c r o f i l a m e n t s .B i o c h e m J 421:l7l-rgi) Y a m a m o t o A , H i r a o k a Y ( 2 0 0 3 ) C y t o p l a s m i cd y n e i n i n f u n g i : i n s i g h t s from nuclearmrgration. J C e l l S c i I l 6 : 4 5 0 1 - 4 5l 2 Zandomeni K' Schopf'erP (1994) Mechanosensory microtubule reorientation in the eprdemis of m a r z e c o l e o p t i l e ss u b j e c t e dt o b e n d i n g s t r e s s .p r o t o p l a s m al g 2 : 9 6 _ l 0 l Zhou J. Wang B. Li Y' Wang Y. Zhu L ll(tol t Responsesof Chrysanthenrrrzr cells to mechanical stimulation require intact microtubules ancl plasma membrane-cell wall adhesion. J plant Growth Regul 26:-55-68 Zimmermann W (196-5)Die Telomtheorie. Fischer. Stutrgarr Z i m m e r m a n n S , N ü m b e r g e r T . F r a c h i s s eJ M . w i r t z w . G u e r n J, Hedrich R, Scheel D (1g97) Receptor-mediated activation of a plant Ca2*-permeable ion channel involved ln palhogen d e f - e n s eP. r o c N a r l A c a c l S c i U S A 9 4 : 2 7 5l _ 2 7 5 5