* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Immunohistochemical description of the endogenous cannabinoid
Axon guidance wikipedia , lookup
Holonomic brain theory wikipedia , lookup
NMDA receptor wikipedia , lookup
Apical dendrite wikipedia , lookup
Synaptic gating wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Optogenetics wikipedia , lookup
Development of the nervous system wikipedia , lookup
Long-term depression wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Aging brain wikipedia , lookup
Synaptogenesis wikipedia , lookup
Subventricular zone wikipedia , lookup
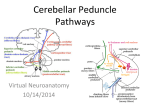
Neuroanatomy wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Signal transduction wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
THE JOURNAL OF COMPARATIVE NEUROLOGY 509:400 – 421 (2008) Immunohistochemical Description of the Endogenous Cannabinoid System in the Rat Cerebellum and Functionally Related Nuclei JUAN SUÁREZ,1* FRANCISCO JAVIER BERMÚDEZ-SILVA,1 KEN MACKIE,2 CATHERINE LEDENT,3 ANDREAS ZIMMER,4 BENJAMIN F. CRAVATT,5 1 AND FERNANDO RODRÍGUEZ DE FONSECA * 1 Laboratorio de Medicina Regenerativa, Fundación IMABIS, 29010 Málaga, Spain 2 Departments of Psychological and Brain Sciences, Indiana University, Bloomington, IN 47401 3 Institut de Recherche Interdisciplinaire en Biologie Humaine et Moléculaire, Université Libre de Bruxelles, B-1050 Bruxelles, Belgium 4 Institute of Molecular Psychiatry, University of Bonn, 53115 Bonn, Germany 5 Chemical Biology and Cell Biology, The Scripps Research Institute, La Jolla, California 92037 ABSTRACT We report a detailed analysis of the distribution of relevant proteins of the endogenous cannabinoid system in the rat cerebellum (cerebellar cortex and deep cerebellar nuclei) and the two functionally related nuclei, the vestibular nuclei and the inferior olive. These proteins include the two main cannabinoid receptors (CB1 and CB2), the enzymes involved in cannabinoid biosynthesis (DAGL␣, DAGL, and NAPE-PLD), and the endocannabinoiddegradating enzymes (FAAH and MAGL). With regard to the cerebellar cortex, these data confirm several published reports on the distribution of cannabinoid CB1 receptors, DAGL␣, MAGL, and FAAH, which suggests a role of endocannabinoids as retrograde messengers in the synapses of the Purkinje cells by either parallel fibers of granule cells or climbing fibers from the inferior olive or GABAergic interneuron. Additionally, we describe the presence of CB2 receptors in fibers related to Purkinje somata (Pinceau formations) and dendrites (parallel fibers), suggesting a potential role of this receptor in the retrograde cannabinoid signaling. A remarkable finding of the present study is the description of the different elements of the endogenous cannabinoid system in both the main afferent nuclei to the cerebellar cortex (the inferior olive) and the efferent cerebellar pathway (the deep cerebellar nuclei). The presence of the endogenous cannabinoid system at this level establishes the basis for endocannabinoid-mediated synaptic plasticity as a control mechanism in motor learning, opening new research lines for the study of the contribution of this system in gait disorders affecting the cerebellum. J. Comp. Neurol. 509:400 – 421, 2008. © 2008 Wiley-Liss, Inc. Indexing terms: endocannabinoid system; cerebellar cortex; deep cerebellar nuclei; vestibular nuclei; inferior olive; CB1 receptor; CB2 receptor; immunohistochemistry This article includes Supplementary Material available via the Internet at http://www.interscience.wiley.com/jpages/0021-9967/suppmat. Grant sponsor: Consejerı́a de Salud (Junta de Andalucı́a); Grant number: PI-0220; MEC; Grant number: SAF 2004/07762; Grant sponsor: Instituto de Salud Carlos III; Grant number: 07/1226; Grant number: 07/0880; Grant sponsor: Plan Nacional Sobre Drogas; Grant sponsor: Consejeria de Innovación Ciencia y Empresa (Junta de Andalucı́a); Grant number: REDES RTA RD06/001; Grant sponsor: 5th Framework Programme; Grant number: TARGALC QLRT-2001-01048. © 2008 WILEY-LISS, INC. *Correspondence to: Juan Suárez and Fernando Rodrı́guez de Fonseca, Laboratorio de Medicina Regenerativa, Fundación IMABIS, Avenida Carlos Haya 82, 29010 Málaga, Spain. E-mail: [email protected]; [email protected] Received 21 February 2007; Revised 14 August 2007; Accepted 28 April 2008 DOI 10.1002/cne.21774 Published online in Wiley InterScience (www.interscience.wiley.com). The Journal of Comparative Neurology ENDOCANNABINOID AND CEREBELLUM 401 Analysis of the CB1 receptor expression in the rat brain by in situ hybridization histochemistry and immunocytochemistry has provided important insights into the functional neuroanatomy of the endocannabinoid system (Matsuda et al., 1993; Pettit et al., 1998; Egertová and Elphick, 2000; Van Sickle et al., 2005; Gong et al., 2006). Electrophysiological studies and the finding of CB1 receptor in the cerebellar synapses suggest that endocannabinoids act as retrograde messengers in the cerebellum. This role of the endocannabinoid system is confirmed by the finding of different types of endocannabinoid-mediated synaptic plasticity, including both short-term [depolarizationinduced suppression of inhibition (DSI) and excitation (DSE)] and the more permanent long-term depression (LTD; for review see Wilson and Nicoll, 2002; Diana and Marty, 2004; Safo and Regehr, 2005). The presence of the endocannabinoid CB1 receptor in the cerebellum implies that the endocannabinoid system plays a central role in real-time regulation of movement and neuroadaptations underlying motor control and motor learning. In fact, relevant pharmacological actions of exogenously administered cannabinoids are ataxia and catalepsy (Rodrı́guez de Fonseca et al., 1998) and modulation of eye blink conditioning (Kishimoto and Kano, 2006; Skosnik et al., 2007). CB1 receptors are located in axon terminals of parallel fibers of cerebellar granular cells and climbing fibers of inferior olive neurons that provide excitatory input on Purkinje cells. In addition, CB1 receptors are located in axon terminals of cerebellar basket and stellate cells providing inhibitory input on Purkinje cells (Mailleux and Vanderhaeghen, 1992; Matsuda et al., 1993; Tsou et al., 1998; Egertová and Elphick, 2000; Cristino et al., 2006; Kawamura et al., 2006). Modulation of excitatory and inhibitory input on Purkinje cells by the endocannabinoid system allows Purkinje cells to refine the output of motor responses from cerebellum. However, so far, little information is available on the presence and function of the cannabinoid CB2 receptor (Skaper et al., 1996; Lu et al., 2000; Zhang et al., 2003; Pazos et al., 2004; Benito et al., 2005; Sheng et al., 2005) and other components of the endocannabinoid system, such as cannabinoid biosynthesis and degradation en- zymes, in the brain (Dihn et al., 2002; Romero et al., 2002; Bisogno et al., 2003; Egertová et al., 2003; Okamoto et al., 2004). Recently, immunohistochemical studies revealed the distribution of CB2 receptor in the rat brain, particularly in cerebellum and hippocampus (Van Sickle et al., 2005; Gong et al., 2006). The finding of CB2 receptor in the cerebellum suggests the need to reevaluate the effects of exogenous and endogenous cannabinoids on neurotransmission. There are some studies demonstrating the presence of the cannabinoid degradation enzymes FAAH (Cravatt et al., 1995, 1996; Egertová et al., 1998; Goparaju et al., 1998; Tsou et al., 1998) and MAGL (Dihn et al., 2002) in the brain. Other studies report a general analysis of FAAH expression in specific neuronal population of mouse and human brain, including cerebellar Purkinje cells, neurons of cerebellar nuclei and inferior olive, neocortical and hippocampal pyramidal neurons, and striatal projecting neurons. These localizations of FAAH suggest a complementary distribution with CB1 expression in these brain regions (Romero et al., 2002; Egertová et al., 2003). Northern blot and in situ hybridization analyses reveal that MAGL is heterogeneously expressed in some brain areas, including hippocampus, cortex, cerebellum, and anterior thalamus, where CB1 receptor is also expressed, indicating a presynaptic localization of the enzyme (Dihn et al., 2002). Indeed, the recent identification of 2-AG and AEA biosynthesis and release enzymes, DAGL␣ and DAGL (Bisogno et al., 2003), and NAPE-PLD (Okamoto et al., 2004) has provided new insights on the endocannabinoid signaling system in the brain. Pharmacological studies suggest that DAGL and NAPE-PLD activity is required for inhibition of ␥-aminobutyric acid (GABA)-ergic transmission by glutamatergic input (Chevaleyre and Castillo, 2003). Additionally, DAGL activity is related to axonal growth and guidance during development (Brittis et al., 1996; Williams et al., 2003). The expression of DAGL isozymes (␣ and  forms) changes during development of the brain; that is, they are expressed in axonal tracts of the embryo and then in dendritic fields of the adult mouse brain (Bisogno et al., 2003). In the adult mouse cerebel- Abbreviations b c CbCx CbN cf DEn DG g G GrL Hi icp IntA IntDL IntDM IntP IntPPC IO IOD IODM IOM IOPr basket cell collaterals cerebellar cortex cerebellar nuclei climbing fibers dorsal endopiriform nucleus dentate gyrus granular cell golgi cell granular layer hippocampus inferior cerebellar peduncle interposed cerebellar nucleus, part anterior interposed cerebellar nucleus, dorsolateral hump interposed cerebellar nucleus, dorsomedial crest interposed cerebellar nucleus, part posterior interposed cerebellar nucleus, posterior parvicellular part inferior olive inferior olive, dorsal nucleus inferior olive, dorsomedial cell group inferior olive, medial nucleus inferior olive, principal nucleus LA Lat LatPC LVe mf Med MedDL ML MVe MVeMC MVePC P pf pif py s scp sp5 SpVe SuVe VeCb VeN lateral amygdaloid nucleus lateral (dentate) cerebellar nucleus lateral cerebellar nucleus, parvicellular part lateral vestibular nucleus mossy fibers medial (fastigial) cerebellar nucleus medial cerebellar nucleus, dorsolateral protuberance molecular layer medial vestibular nucleus medial vestibular nucleus, magnocellular part medial vestibular nucleus, parvicellular part purkinje cell parallel fibers pinceau formation pyramidal tract superficial stellate cell superior cerebellar peduncle spinal trigeminal tract spinal vestibular nucleus superior vestibular nucleus vestibulocerebellar nucleus vestibular nuclei The Journal of Comparative Neurology 402 lum, the substantial down-regulation of DAGL contrasts with the strong staining of DAGL␣ in the Purkinje cell dendritic field (Bisogno et al., 2003). For this study, we selected NAPE-PLD as the anandamide-synthesizing enzyme. A recent paper described the molecular characterization of NAPE-PLD; its authors noted the presence of NAPE-PLD activity in mouse brain, including the cerebellum (Okamoto et al., 2004). We have also detected NAPE-PLD expression in rat cerebellum (Ferrer et al., 2007). However, its immunohistochemical localization in the brain has not been analyzed so far. It is important to note that NAPE-PLD is not the only source for anandamide in the brain (Leung et al., 2006) but is the first of a series of enzymes capable of generating anandamide from the membrane precursor N-arachidonyl-phosphatidyl ethanolamide (NAPE), such as ␣/ hydrolase 4, lyso-PLD, lyso-PLC, and phosphatases such as PTPN22 (Leung et al., 2006; Simon and Cravatt, 2006; Liu et al., 2007). However, despite discrepancies in substrate specificity and the lack of specific test for the activation of NAPE-PLD in neural circuits (Liu et al., 2007), NAPE-PLD remains an important source of anandamide in the brain. Therefore, the molecular characterization of new synthesis pathways for anandamide in the brain will determine this important aspect of endocannabinoid physiology. The aim of this study was to determine the distribution of the endocannabinoid receptors CB1 and CB2 and the endocannabinoid biosynthesis and degradation enzymes DAGL␣, DAGL, NAPE-PLD, FAAH, and MAGL by immunohistochemistry in the rat cerebellum (cerebellar cortex and cerebellar nuclei) and other functionally related brain areas, such as vestibular nuclei and inferior olive. The exact localization of these cannabinoid enzymes and receptors in the rat cerebellum may facilitate a neuroanatomical framework for the analysis of the physiological roles of the endocannabinoid signaling system. MATERIAL AND METHODS Generation of NAPE-PLD-, DAGL␣-, DAGL-, and MAGL-specific antibodies We have generated polyclonal rabbit antibodies against proteins of the cannabinoid machinery. Immunizing peptides were 1) a 13-amino-acid (aa) peptide comprising part of both the C-terminal and the N-terminal regions of NAPE-PLD (MDENSCDKAFEET); 2) a 16-aa peptide from the C-terminal region of DAGL␣ (CGASPTKQDDLVISAR); 3) a 16-aa peptide from an internal sequence of DAGL (SSDSPLDSPTKYPTLC); 4) a 15-aa peptide from the N-terminal region of MAGL (SSPRRTPQNVPYQDL); 5) a 73-aa peptide (401– 473) from the C-terminal region of CB1 receptor; and 6) a 14-aa peptide (328 –342) from the C-terminal region of CB2 receptor. We employed a chimeric sequence peptide as immunogen for NAPE-PLD antibody generation. The aim of this chimeric construction was to contain two distant epitopes exposed in the native protein because one of them belongs to the N-terminal and the other to the C-terminal region of the protein, both regions having random coil structures. NAPE-PLD, DAGL␣, and DAGL peptides were synthesized and coupled to keyhole limpet hemocyanin (KLH; JPT Peptide Technologies, Berlin, Germany). The three peptides were injected into rabbits (two animals per peptide), according J. SUÁREZ ET AL. to standard protocols for generation of antisera, with the IgG fraction subsequently purified by means of a protein A column (Sigma, St. Louis, MO). MAGL antibody was produced in the laboratory of Dr. D. Piomelli (Dihn et al., 2002). MAGL peptide was synthesized and coupled to KLH by addition of a cysteine at the peptide N terminus (United Biochemical, Seattle, WA). The conjugated peptide was injected into two rabbits to generate antisera (Strategic Biosolutions, Ramona, CA). The peptide was then conjugated to an agarose column and the antiserum purified according to the manufacturer’s instructions (AminoLink; Pierce Endogen, Rockford, IL). For CB1 and CB2 antibody generation, we injected rabbits with a fusion protein composed of glutathione-S transferase (GST) and CB1 residues 401– 473 or CB2 residues 328 –342, using conventional techniques. Polyclonal antisera and purified antibodies specific for CB1 and CB2 were collected by affinity chromatography against both GST and immunizing fusion protein. Immunohistochemistry We have evaluated the presence of CB1 and CB2 receptors, FAAH, MAGL, DAGL␣, DAGL, and NAPE-PLD in the adult rat cerebellum by immunohistochemistry. Manipulation of animals was in accordance with the European Communities Council Directives (86/609/EEC) on the treatment of experimental animals. Adult Wistar rats (n ⫽ 6; 300 g) were deeply anesthetized with 2,2,2-tribromoethanol (300 mg/kg i.p.) and briefly transcardially perfused with 0.1 M phosphatebuffered saline (PBS; pH 7.4), followed by 4% paraformaldehyde in PBS at 4°C for 30 minutes. Brains were dissected, postfixed overnight in buffered paraformaldehyde at 4°C, equilibrated with 30% sucrose in PBS at 4°C, frozen, and cut into 40-m-thick-transverse or sagittal sections using a sliding microtome. We then collected 19 alternate series of sections from each rat brain to process the seven antibodies and Nissl staining. Free-floating sections were first incubated in H2O distilled containing 50 mM sodium citrate (pH 9) for 30 minutes at 80°C, followed by several washes in PBS. Then, we incubated sections with 3% hydrogen peroxide in PBS for 20 minutes at room temperature to inhibit endogenous peroxidase, followed by three times in PBS. We have used seven primary antibodies. The anti-CB1 was developed in rabbits by using a fusion protein as immunogen containing 73 amino acid residues (401– 473) of the rat CB1 receptor (Wager-Miller et al., 2002); the anti-CB2 was produced in rabbits by using a fusion protein containing 14 amino acid residues (328 –342) from rat CB2 receptor; the anti-FAAH was developed in rabbits by using a synthetic peptide corresponding to 561–579 amino acid fragment of rat fatty acid amine hydrolase conjugated to KLH as immunogen (Cayman Chemical; catalog No. 101600, lot. No. 157878). The anti-NAPE-PLD, antiDAGL␣, anti-DAGL, and anti-MAGL were developed in rabbits as described above. Sections were incubated in the diluted primary antibody (anti-CB1, diluted 1:500; antirat CB2, 1:500; anti-rat FAAH, 1:200; anti-MAGL, 1:200; anti-DAGL␣, 1:500; anti-DAGL, 1:200; and anti-NAPEPLD, 1:400) overnight at room temperature. After three washes in PBS, the sections were incubated in a biotinylated donkey anti-rabbit immunoglobulin (Amersham, Little Chalfont, England) diluted 1:500 for 1 hour, washed again in PBS, and incubated in ExtrAvidin peroxidase The Journal of Comparative Neurology ENDOCANNABINOID AND CEREBELLUM (Sigma) diluted 1:2,000 for 1 hour. We revealed immunolabeling with 0.05% diaminobenzidine (DAB; Sigma), 0.05% nickel ammonium sulfate, and 0.03% H2O2 in PBS. All steps were carried out with gentle agitation at room temperature. After the sections had been washed in PBS, they were mounted on gelatinized slides, air dried, dehydrated in ethanol, cleared in xylene, and coverslipped with Eukitt mounting medium (Kindler GmBH and Co., Freiburg, Germany). Digital photographs were taken on an Olympus BX41 microscope equipped with an Olympus DP70 digital camera. Digital images were adjusted for brightness/contrast in Adobe Photoshop (Adobe, San Jose, CA), and the figures were mounted and labelled in Adobe PageMaker. Antibody specificity and controls We performed Western blot analyses to demonstrate that CB1, CB2, FAAH, MAGL, DAGL␣, DAGL, and NAPE-PLD antibodies recognized the corresponding antigen in the rat cerebellum. To perform Western blot analysis, we used fresh tissue from Wistar male rats. Animals were killed by 2,2,2-tribromoethanol (Fluka, Steinheim, Germany), and the cerebellum was immediately isolated, snap frozen in liquid nitrogen, and stored at – 80°C until use. Membrane extracts of rat cerebellum were prepared in HEPES 50 mM (pH 8)-sucrose 0.32 M buffer by using a homogenizer. The homogenate was centrifuged at 800g for 10 minutes at 4°C and the supernatant centrifuged at 40,000g for 30 minutes. We resuspended the pellets in HEPES 50 mM buffer and potterized using a homogenizer. For immunoblotting, equivalent amounts of membrane proteins (45 g) from rat cerebellum were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), electroblotted onto nitrocellulose membranes, and controlled by Ponceau red staining. For DAGL immunoblotting, we used stringent conditions with the addition of dithiothreitol 0.15 mM in the sample buffer. We preincubated blots with a blocking buffer containing PBS, 0.1% Tween 20, and 2% albumin fraction V from bovine serum (Merck) at room temperature for 1 hour. For protein detection, each blotted membrane lane was incubated separately with the specific CB1 (1:250), CB2 (1:250), FAAH (1:100), MAGL (1:200), DAGL␣ (1: 300), DAGL (1:200), and NAPE-PLD (1:400) antibodies and diluted in PBS containing 0.1% Tween 20 and 2% albumin fraction V from bovine serum at room temperature overnight. After extensive washing in PBS containing 1% Tween 20 (PBS-T), a peroxydase-conjugated goat antirabbit antibody (Promega, Madison, WI) was added (1: 2,500) for 1 hour at room temperature. Biotinylated marker proteins with defined molecular weights were used for molecular weight determination in Western blots (ECL Western Blotting Molecular Weight Markers; Amersham). We incubated the corresponding markers lane with ExtrAvidin peroxidase (Sigma). Membranes were then subjected to repeated washing in PBS-T and the specific protein bands visualized by using the enhanced chemiluminiscence technique (ECL; Amersham) and Auto-Biochemi Imaging System (LTF Labortechnik GmbH, Wasserburg/Bodensee, Germany). Western Blots showed that each primary antibody detected a protein of the expected molecular size (see Fig. 1A). As additional controls, cerebellar and hippocampal sections from CB1 receptor knockout mice (Ledent et al., 403 1999), CB2 receptor knockout mice (Buckley et al., 2000), NAPE-PLD knockout mice (Cravatt et al., 2001), and wildtype controls (n ⫽ 2 pairs) were also analyzed. Immunohistochemical protocol was carried out as described above (anti-CB1, diluted 1:500; anti-rat CB2, 1:500; anti-rat NAPE-PLD, 1:400). We observed that immunostaining was almost completely absent in CB1 knockout mouse brain, but weak staining was found in the spinal trigeminal tract at cerebellar levels and in the cerebral peduncle at hippocampal levels (Suppl. Fig. 1). With the exception of these features, all of the staining in wild-type brain is specifically attributable to CB1 expression. We did not observed labeling in the CB2 receptor knockout mouse or NAPE-PLD receptor knockout mouse, whereas the wildtype mouse showed labelling similar to that of the rat brain (Suppl. Figs. 2, 3). We also incubated blotted membrane lanes with the primary antibody preadsorbed with the immunizing peptide CB1 (10 g/ml), CB2 (10 g/ml), FAAH (20 g/ml; Cayman, Ann Harbor, MI), MAGL (13 g/ml; kindly donated by Dr. D. Piomelli), DAGL␣ (175 g/ml; JPT; see above), DAGL (32 g/ml; JPT; see above), and NAPEPLD (16 g/ml; JPT; see above) or incubated by replacing the primary antiserum by 2% albumin fraction V from bovine serum (see Fig. 1A). In addition, we incubated brain sections with the primary antibody preadsorbed with the immunizing peptide under the same conditions as described above. We did not detect staining under these conditions (Suppl. Figs. 4, 5). RESULTS In the present study, we mapped the expression of the cannabinoid receptors CB1 and CB2; the degradation enzymes FAAH and MAGL; and the synthesis enzymes DAGL␣, DAGL, and NAPE-PLD in the rat cerebellum and two main cerebellar-related areas, vestibular nuclei and inferior olive. The analysis of the immunostaining patterns was carried out at transverse and sagittal planes of the adult rat brain by comparing it with the cytoarchitecture and with published data on its neurochemistry and connections. The intensity of the immunoreactivity for each antibody was very similar in all the six rat brains used in this study. Numerous brain regions such as hippocampus, basal ganglia, substantia nigra, and cerebellum intensely expressed CB1 receptors, FAAH, MAGL, and DAGL␣, in comparison with CB2 receptors, DAGL, and NAPE-PLD expression. The nomenclature for nuclei and subdivisions referred to in the present study is widely accepted and used in the rat brain atlas by Paxinos and Watson (1998). Results for this study are described in the text and summarized in a rating scale (Table 1). Grayscale values measured in single cerebellar, vestibular, and olive nuclei are represented on an arbitrary scale of four labelling intensities, from “⫹,” meaning “very low” (slightly above the density measured in sections incubated in preadsorbed antibody; see Suppl. Figs. 4, 5) to “⫹⫹⫹⫹,” meaning “very high” (according to the highest signal density in the specimen, i.e., parvicellular part of the medial vestibular nucleus). Previously, we analyzed controls and Western blot results to demonstrate that CB1, CB2, FAAH, MAGL, DAGL␣, DAGL, and NAPE-PLD antibodies recognize the corresponding antigen in the rat cerebellum. The Journal of Comparative Neurology 404 J. SUÁREZ ET AL. TABLE 1. Immunoreactivity in Cerebellum, Vestibular Nuclei, and Inferior Olive1 CB1 c Cerebellar cortex Molecular layer Granular layer Purkinje cells Cerebellar nuclei IntA IntP IntPPC IntDL IntDM Lat LatPC Med MedDL Vestibular nuclei LVe MVeMC MVePC SpVe SuVe VeCb Inferior olive – – CB2 f c ⫹⫹⫹⫹ ⫹⫹ – – – FAAH f c ⫹⫹ ⫹⫹ – ⫹ – MAGL f c ⫹⫹⫹⫹ ⫹ ⫹⫹ ⫹⫹⫹⫹ ⫹⫹⫹⫹ ⫹⫹ DAGL␣ f c ⫹⫹⫹ ⫹⫹ – – DAGL f c ⫹⫹⫹⫹ ⫹⫹ – ⫹ ⫹ – – – – – – – – – ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ – – – – – – – – – ⫹⫹⫹ ⫹⫹⫹ ⫹⫹⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹ ⫹ – ⫹⫹ ⫹ ⫹ – ⫹⫹ ⫹⫹ ⫹⫹⫹⫹ ⫹⫹⫹⫹ ⫹⫹⫹⫹ ⫹⫹⫹⫹ ⫹⫹⫹⫹ ⫹⫹⫹⫹ ⫹⫹⫹⫹ ⫹⫹⫹⫹ ⫹⫹⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ – – – – – – – – – ⫹⫹ ⫹⫹ ⫹⫹⫹⫹ ⫹⫹ ⫹⫹ ⫹⫹ ⫹⫹⫹⫹ ⫹⫹ ⫹⫹ ⫹⫹ ⫹⫹ ⫹ ⫹⫹ ⫹ ⫹⫹ ⫹ ⫹⫹ ⫹⫹ ⫹⫹⫹ – – – – – – ⫹⫹ ⫹⫹⫹ ⫹ ⫹⫹⫹ ⫹ ⫹ ⫹⫹ – – – – – – – ⫹⫹⫹ ⫹⫹⫹ ⫹⫹⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹⫹ ⫹ ⫹⫹ ⫹ ⫹ – ⫹⫹ ⫹⫹⫹⫹ ⫹ ⫹ ⫹ ⫹⫹⫹⫹ ⫹⫹ ⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹⫹⫹⫹ ⫹⫹ ⫹⫹ ⫹⫹ ⫹ ⫹ ⫹ ⫹⫹ ⫹ ⫹⫹ ⫹ ⫹⫹ – – – – – – – ⫹⫹⫹ ⫹⫹ ⫹⫹⫹⫹ ⫹⫹ ⫹⫹ ⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹⫹ ⫹⫹⫹ ⫹⫹ ⫹⫹ ⫹ ⫹⫹ f ⫹⫹⫹ ⫹⫹ ⫹⫹⫹ NAPE-PLD c ⫹ ⫹⫹ f ⫹⫹⫹ ⫹⫹⫹ ⫹ ⫹⫹ ⫹⫹ ⫹⫹ ⫹⫹ ⫹⫹ ⫹⫹ ⫹⫹ ⫹⫹ ⫹⫹ ⫹⫹ ⫹⫹ ⫹ ⫹⫹ ⫹⫹ ⫹⫹ ⫹ ⫹⫹ ⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹⫹ ⫹ ⫹⫹⫹ ⫹ ⫹⫹ ⫹⫹ ⫹ ⫹⫹⫹ ⫹⫹ ⫹⫹⫹ ⫹⫹ ⫹⫹ ⫹ ⫹ ⫹⫹ ⫹⫹ ⫹⫹⫹ ⫹ ⫹⫹ ⫹⫹ ⫹⫹ 1 Rating scale of the immunoreactivity of each structure in cells (c) and fibers (f). Symbols are as follows: very high (⫹⫹⫹⫹), high (⫹⫹⫹), low (⫹⫹), very low (⫹), and without immunoreactivity (–). Western blot analysis Western blot analyses of membrane extracts from rat cerebellum revealed CB1 immunostaining as a prominent band at about 60 kD (Fig. 1A, lane 1). CB2 immunostaining also showed a prominent band at about 55 kD (Fig. 1A, lane 7). Immunoblots for FAAH and NAPE-PLD revealed a single band with a molecular mass of 63 and 46 kD, respectively (Fig. 1A, lanes 3 and 5, respectively). In addition, under more stringent conditions, DAGL immunoblotting showed a prominent band of 76 kD and another, less intense one of 97 kD (Fig. 1A, lane 11). DAGL␣ immunoblotting showed an expected band of 120 kD (Fig. 1A, lane 13). Analysis of MAGL immunoreactivity confirmed two bands of 37 and 35 kD, but we also observed an additional band at about 62 kD (Fig. 1A, lane 9). In all cases, the immunoreactive bands were abolished after absorption with the immunizing peptides (Fig. 1A, lanes 2, 4, 6, 8, 10, 12, 14). CB1 immunoreactivity We observed a prominent fiber CB1 labelling surrounding Purkinje somata and proximal axons that may consist of clustered basket cell axons, called Pinceau formations (Fig. 2B, inset). Numerous immunostained fibers are also widespread throughout the branches of the cerebellar white matter and well-defined fibers (but not mossy fibers) dispersed from the granular layer into the molecular layer (Fig. 2B, inset). Therefore, these fibers may represent axons from inferior olive (climbing fibers). We clearly distinguished the molecular layer of cerebellar cortex by an intense CB1 immunoreactivity consisting of a dense network of immunostaining fibers and puncta, which may principally correspond to climbing fiber terminals but also parallel fibers from granular cells. The climbing fibers represented the main external Purkinje afferent innervations originating from the inferior olive (see Fig. 10B). We did not observed stained cell bodies in the cerebellar cortex. Cerebellar and vestibular nuclei present a very low CB1 immunoreactivity (Fig. 2A,C–F). In general, our results showed that all cerebellar nuclei (medial, lateral, and interposed nuclei) contained very weak neuropil. However, it should be noted that numerous fiber tracts crossed the cerebellar nuclei, which could be distinguished by their position and orientation. Some of these fiber bundles crossed unstained peduncles and formed part of the superior cerebellar peduncle (Fig. 2C,D). Therefore, part of these fibers may represent axons from cerebellar nuclei that projected to red nuclei, dorsal thalamus, and motor cortex. Other peduncles of CB1⫹ fibers coursed dorsally from the inferior cerebellar peduncle to the cerebellar cortex. Most of these fibers constituted axons from the inferior olive, in agreement with the presence of climbing fibers in the molecular layer (Fig. 2E). An intense CB1 immunoreactivity consisting of a network of neuropil and puncta characterized the dorsal portion of the principal nucleus of the inferior olive (IOPr; see Fig. 9A). The presence of numerous CB1⫹ fibers in the rubroolivary tract (data not shown) suggested that most of the fiber terminals in the IOPr originated from the red nucleus. The rest of the inferior olivary complex showed very low immunoreactivity. Most vestibular nuclei also presented very weak CB1 immunoreactivity (Fig. 2C). We distinguished spinal vestibular nucleus (SpVe) and the magnocellular part of medial vestibular nucleus (MVeMC) from its parvocellular part (MVePC) by a stronger CB1⫹ neuropil (Fig. 2C). It is worth noticing that typical giant neurons of the lateral vestibular nuclei (LVe) were moderately CB1 immunostained, whereas their dendritic initial segments could be clearly distinguished (Fig. 2E). CB2 immunoreactivity The distribution of CB2 immunoreactivity in the cerebellar cortex was similar to that of CB1 immunoreactivity. As occurred with CB1 immunoreactivity, we observed CB2-immunoreactive (CB2⫹) fiber terminals surrounding Purkinje somata and proximal axons (Pinceau formations). Purkinje cells were not CB2 immunoreactive. The The Journal of Comparative Neurology ENDOCANNABINOID AND CEREBELLUM 405 Fig. 1. A: Western blots of membrane extracts from rat cerebellum show prominent immunoreactive bands of expected molecular masses of 60 kD for CB1; 63 kD for FAAH; 46 kD for NAPE-PLD; 55 kD for CB2; 62, 37, and 35 kD for MAGL; 97 and 76 kD for DAGL; and 120 kD for DAGL␣. Positions of molecular markers (MW) are indicated at left. B: Major biochemical pathways for endogenous cannabinoid signalling system. Anandamide (AEA) is released from a membrane lipid precursor, N-arachidonoyl-phosphatidylethanolamine (NAPE), by the action of a specific phospholipase D (PLD) activated by depolarization, postsynaptic calcium increases, or G-protein-coupled receptor stimulation. The membrane enzyme N-acyltransferase (NAT) catalyses NAPE biosynthesis, which transfers arachidonic acid from phosphatidylcholine (PhChol) to the head group of phosphatidylethanolamine (PhEth). Postsynaptic calcium influx and the activation of metabotropic receptors coupled to phosphatidyl-inositol-specific phospholipase C (PLC) and diacylglycerol (DAG) lipase pathway lead to increases in 2-arachidonoylglycerol (2-AG) production. Endocannabinoid signalling includes uptake into cells mediated by a transporter (AT) and hydrolysis by two specific enzymatic systems, the fatty acid amide hydrolase (FAAH) and the monoacylglyceride lipase (MAGL). molecular layer also contained a dense network of immunostained fibers and neuropil, extending from the granular layer to the pial surface of the molecular layer. Most CB2⫹ fibers of the molecular layer were disposed in parallel and presented numerous varicosities along their surface. Therefore, these fibers may principally represent parallel fibers originating from granular cells (Fig. 3B, inset). In contrast to CB1 immunoreactivity, the granular layer showed moderate neuropil CB2 immunoreactivity. These fibers showed a mossy aspect (mossy fibers) and so probably may represent axons from pontine nuclei, vestibular nuclei, and spinal cord (Fig. 3B). In addition, all subdivisions of the inferior olive showed a strong CB2 immunoreactivity that consisted of a dense network of fibers (Fig. 9B). The main olivary afferent innervations originated from the spinal cord, so these fiber terminals in the IO may constitute collaterals of the mossy fibers in the cerebellar cortex from the spinal cord. The CB2 immunoreactivity in the cerebellar and vestibular nuclei was different from the CB1 immunoreactivity, especially the prominent neuropil immunoreactivity (Fig. 3C–F). Most cerebellar nuclei are characterized by a dense network of CB2⫹ neuropil and fiber terminals that define numerous unstained fiber bundles and cell profiles (Fig. 3D,F). These CB2⫹ fibers may have the same origin as the CB2⫹ mossy fibers in the granular layer of the vestibular nuclei, pontine nuclei, and spinal cord, but they may also constitute projections from Purkinje cells. A stronger neuropil immunostaining also characterized vestibular nuclei such as MVePC and SpVe (Fig. 3C,E). Most fiber termi- The Journal of Comparative Neurology 406 Fig. 2. CB1 immunoreactivity in the rat cerebellum and vestibular nuclei. A: General view of a coronal section through the cerebellum and vestibular nuclei. B: High-magnification photomicrographs of the cerebellar cortex showing clustered basket cell axons (pinceau formations) surrounding unstained Purkinje somata (inset). Numerous fibers fill the branches of the cerebellar white matter and disperse from the granular layer into the molecular layer (arrowhead in inset). J. SUÁREZ ET AL. C: Low-magnification photomicrograph showing CB1 immunoreactivity throughout the cerebellar and vestibular nuclei. Note fiber tracts that cross the cerebellar nuclei and can be distinguished by their position and orientation into the superior (D) and inferior (E) cerebellar peduncle and the giant neurons in LVe (F). For abbreviations see list. Scale bars ⫽ 1 mm in A; 200 m in B,C; 100 m in D–F; 20 m in inset. The Journal of Comparative Neurology ENDOCANNABINOID AND CEREBELLUM Fig. 3. CB2 immunoreactivity in the rat cerebellum and vestibular nuclei. A: General view of a coronal section through the cerebellum and vestibular nuclei. B: Detail of a transverse section of the cerebellar cortex showing mossy fibers in the granular layer, parallel fibers in the molecular layer (arrows), and “pinceau” formations in the Purkinje layer (arrowheads). Note the unstained Purkinje somata (in- 407 set). C: Low-magnification photomicrograph showing CB2 immunoreactivity throughout the cerebellar and vestibular nuclei. Note cell profiles immersed in a dense fiber network in all cerebellar nuclei (D,E) and the intense neuropil immunoreactivity in the MVe (F). For abbreviations see list. Scale bars ⫽ 1 mm in A; 100 m in B,E,F; 200 m in C; 50 m in D; 20 m in inset. The Journal of Comparative Neurology 408 J. SUÁREZ ET AL. nals in the vestibular nuclei may constitute projections from Purkinje cells. FAAH immunoreactivity We clearly observed FAAH immunoreactivity in Purkinje neurons, which showed intensely stained cell bodies and a dense network of fibers in the molecular layer that, in sagittal sections, characterized the dendrite tree of the Purkinje cells (Fig. 4B, inset). On the other hand, we observed an evident FAAH immunoreactivity in a subpopulation of granular cells (Fig. 4B) and in numerous neurons of IO (Fig. 9C). Therefore, we can expect that some of the FAAH-immunoreactive (FAAH⫹) puncta of the molecular layer probably represent climbing and parallel fiber terminals. Poor neuropil immunoreactivity in the granular layer was similar to that of fibers in the cerebellar white matter. The strong FAAH immunoreactivity observed in all cerebellar nuclei was related mainly to the presence of a dense meshwork of fibers, consisting of FAAH⫹ punctate labelling that contained immunoreactive cell bodies (Fig. 4C,D). Most likely, these fibers represented dendritic fibers. Note the moderate density of FAAH⫹ neurons in the IntDL, MeDL, and Med (Fig. 4C,D) and the strongly FAAH⫹ fibers in all cerebellar nuclei (Fig. 4C). However, we observed that most of the vestibular nuclei presented a low immunoreactivity for FAAH (Fig. 4C,E,F), with the exception of LVe, which showed immunoreactivity similar to that of cerebellar nuclei. LVe staining consisted of a dense network of fibers disposed between tracts of the juxtarestiform body along with a number of giants FAAH⫹ neurons spreading from LVe to SpVe (Fig. 4E). Both parts (parvicellular and magnocellular) of the medial vestibular nucleus showed a very low FAAH immunostaining consisting of a number of FAAH⫹ cells embedded in a very poorly stained neuropil (Fig. 4F). MAGL immunoreactivity MAGL immunostaining of the cortex cerebellum showed a distinct pattern in the cerebellar layers (Fig. 5A). The molecular layer was distinguished by its moderate MAGL immunoreactivity consisting of a number of small cells that likely represented basket cells and superficial stellate cells, clearly discernable within a moderately MAGL-immunoreactive (MAGL⫹) neuropil (Fig. 5B). Most MAGL⫹ neuropil in the molecular layer seemed to represent parallel fiber terminals from the granular cells (Fig. 5B) and also probably climbing fibers from IO. Purkinje cell bodies were moderately stained, whereas Purkinje dendrites were not immunoreactive (Fig. 5B, arrowheads). A remarkable feature was the MAGL immunoreactivity of the granular layer, related mainly to the presence of densely packed, well-stained granular cells and possibly others cell types embedded in a moderate neuropil labelling (Fig. 5B). Part of this neuropil likely consisted of mossy fibers from spinal, pontine, and vestibular nuclei. The outer one-third of the granular layer (with respect to its radial dimension to the pial surface) contained dispersed cells with a stronger staining that likely represented Golgi cells according to their unique location under the Purkinje layer (Fig. 5B, inset). Cerebellar and vestibular nuclei presented numerous strongly stained MAGL⫹ neurons, showing a perikaryal and dendritic Golgi-like labelling (Fig. 5C–F). Mediumsized neurons characterized most cerebellar nuclei based on their morphology and orientation (Fig. 5D). However, IntDM, IntPPC, and LatPC showed small MAGL⫹ cells (Fig. 5C,D). LVe and SpVe also presented numerous giant neurons (more dispersed in SpVe) showing a prominent Golgi-like labelling (Fig. 5E). MVePC showed numerous small MAGL⫹ neurons embedded in a moderately immunoreactive neuropil, in contrast to the large neurons in MVeMC (Fig. 5F). All IO subdivisions presented numerous small MAGL⫹ neurons showing less staining than those of the Golgi-like labelling of surrounding areas (Fig. 9D). DAGL␣ immunoreactivity In contrast to MAGL immunoreactivity, the expression of DAGL␣ was particularly prominent in the molecular layer that clearly corresponded to the dendritic field of the Purkinje cells (Fig. 6A,B). Note the weak DAGL␣ immunoreactivity Purkinje cell bodies and the numerous varicosities along the dendritic fibers in transverse and sagittal views (Fig. 6B, insets b⬘,b⬘⬘, respectively). However, the lack of DAGL␣⫹ cells in the granular layer and in all subdivisions of IO (see Fig. 9E) suggests that parallel and climbing fibers did not present DAGL␣ immunoreactivity. We also observed typically DAGL␣⫹ mossy fibers that coursed along the branches of the cerebellar white matter and spread into the granular layer, showing a moderately immunoreactive neuropil (Fig. 6B). However, we did not observe DAGL␣ immunoreactivity in cell bodies of cerebellar and vestibular nuclei (Fig. 6C–F) or in any other region with mossy fiber projections in the granular layer such as the pontine nuclei or the spinal cord (data not shown). Cerebellar and vestibular nuclei presented moderate DAGL␣ immunoreactivity that consisted of a conspicuous network of neuropil and puncta (Fig. 6C–F). In some cerebellar and vestibular regions, the dense neuropil defined numerous profiles of unstained cell bodies (Fig. 6D,F). Of relevance, IntPPC, LatPC, and MVePC were characterized by a prominent neuropil DAGL␣ immunoreactivity (Fig. 6D,F). Additionally, IO was also characterized by the presence of intense neuropil immunoreactivity in contrast to that of surrounding areas (Fig. 9E). DAGL immunoreactivity DAGL immunostaining in the cerebellar cortex was considerably less pronounced than that of DAGL␣ (Fig. 7A,B). We also observed stained DAGL⫹ Purkinje cell bodies and a moderately DAGL immunoreactivity in the molecular layer that may be consistent with the immunohistochemical description by Bisogno and collaborators (2003) for mouse cerebellum (Fig. 7B). In the granular layer, DAGL immunoreactivity was associated mainly with the presence of immunoreactive neuropil but was weaker than that of the molecular layer and some dispersed granular cells (Fig. 7B). Additionally, IO contained abundant DAGL⫹ neurons (Fig. 9F), so DAGL⫹ neuropil of the molecular layer probably contained parallel and climbing fibers from granular cells and IO neurons, respectively, in contrast to the DAGL␣ immunoreactivity. DAGL immunoreactivity in the cerebellar and vestibular nuclei was associated mainly with cell bodies embedded in a network of fibers, in contrast to DAGL␣ immunoreactivity (Fig. 7C). Most cerebellar nuclei presented a DAGL⫹ neuronal distribution similar to that of MAGL⫹ neurons, which is in medium-sized cell bodies homog- The Journal of Comparative Neurology ENDOCANNABINOID AND CEREBELLUM Fig. 4. FAAH immunoreactivity in the rat cerebellum and vestibular nuclei. A: General view of a coronal section through the cerebellum and vestibular nuclei. B: High-magnification photomicrographs of the cerebellar cortex showing intensely stained Purkinje cell bodies and the dense network of Purkinje dendritic fibers (inset in B) and dispersed granular cells (arrowheads). C: Low-magnification photomi- 409 crograph showing FAAH immunoreactivity throughout the cerebellar and vestibular nuclei. Note the low density of FAAH⫹ neurons in the MeDL (D) and the weak immunostaining of the giant neurons in the LVe (E) and the small neurons of the MVePC (F). For abbreviations see list. Scale bars ⫽ 1 mm in A; 100 m in B,D–F; 200 m in C; 20 m in inset. The Journal of Comparative Neurology 410 Fig. 5. MAGL immunoreactivity in the rat cerebellum and vestibular nuclei. A: General view of a coronal section through the cerebellum and vestibular nuclei. B: High-magnification photomicrographs of the cerebellar cortex showing the high density of granular cells, Purkinje somata, and basket and stellate cells homogeneously distributed in the molecular layer. Purkinje dendrites are not immunostained (arrowheads). Note the prominent immunoreactivity of a neuronal subpopulation into the granular layer that, by its position under J. SUÁREZ ET AL. the Purkinje layer, may correspond to Golgi cells (inset in B). C: Lowmagnification photomicrograph showing MAGL immunoreactivity throughout the cerebellar and vestibular nuclei. Note the Golgi-like labelling of the medium-sized neurons in IntP, IntA, and Lat (D) and the small neurons in IntPPC (D) and MVePC (F). Giant neurons of the LVe and large neurons of the MVeMC are also intensely immunoreactive (E,F). For abbreviations see list. Scale bars ⫽ 1 mm in A; 100 m in B,D–F; 200 m in C; 20 m in inset. The Journal of Comparative Neurology ENDOCANNABINOID AND CEREBELLUM Fig. 6. DAGL␣ immunoreactivity in the rat cerebellum and vestibular nuclei. A: General view of a coronal section through the cerebellum and vestibular nuclei. B: High-magnification photomicrographs of the cerebellar cortex showing the Purkinje somata and the dense network of Purkinje dendritic fibers in the molecular layer. Note the numerous varicosities along the dendritic fibers in trans- 411 verse (bⴕ) and sagittal (bⴕⴕ) views. C: Low-magnification photomicrograph showing an intense DAGL␣ immunoreactivity in a network of fibers throughout the cerebellar and vestibular nuclei. Note the higher density of fibers in the IntPPC, LatPC, and MVePC. Scale bars ⫽ 1 mm in A; 100 m in B,D–F; 200 m in C; 20 m in b⬘; 10 m in b⬘⬘. The Journal of Comparative Neurology 412 Fig. 7. DAGL immunoreactivity in the rat cerebellum and vestibular nuclei. A: General view of a coronal section through the cerebellum and vestibular nuclei. Stained DAGL⫹ Purkinje somata and scattered DAGL⫹ granular cells are observed in the cerebellar cortex (B, arrowheads). C: Low-magnification photomicrograph showing J. SUÁREZ ET AL. DAGL immunoreactivity throughout the cerebellar and vestibular nuclei. Note the small neurons in the IntDM (D) and MVePC (F), in comparison with the larger neurons in the IntA (D) and MVeMC (F), and the giant neurons in LVe (E). For abbreviations see list. Scale bars ⫽ 1 mm in A; 100 m in B,D–F; 200 m in C. The Journal of Comparative Neurology ENDOCANNABINOID AND CEREBELLUM enously distributed (Fig. 7C,D). However, fewer small DAGL⫹ neurons were observed in IntPPC and parts of LatPC and IntDM compared with the remaining parts of cerebellar nuclei (Fig. 7C,D). Giant DAGL⫹ neurons characterized LVe (Fig. 7E), whereas small DAGL⫹ neurons immersed in a dense immunoreactive neuropil characterized MVePC (Fig. 7F). NAPE-PLD immunoreactivity The distribution of the NAPE-PLD immunoreactivity was quite different from that described above for CB1 in the cerebellar cortex (Fig. 8A,B). The molecular layer was characterized by a moderately immunoreactive neuropil, in contrast to the considerably weaker immunostaining of the granular layer, which was partially related to dendritic fiber from the strongly stained Purkinje cell bodies (Fig. 8B, inset) but also possibly to parallel fibers from a number of granular cells showing NAPE-PLD immunoreactivity (Fig. 8B). In addition, most IO subdivisions presented a number of weakly NAPE-PLD-immunoreactive (NAPE-PLD⫹) neurons (Fig. 9G), so the molecular layer of the cerebellar cortex could also contain NAPE-PLD⫹ climbing fibers. Note the weakly stained NAPE-PLD⫹ cells in the molecular layer, which may correspond to basket and stellate cells (Fig. 8B, inset). The distribution of NAPE-PLD immunoreactivity in the cerebellar and vestibular nuclei was quite similar to that of DAGL immunoreactivity (Figs. 7C, 8C). Most cerebellar nuclei consisted of intense neuropil immunoreactivity and a number of moderately labelled NAPE-PLD⫹ neurons (Fig. 8C,D). As with DAGL immunoreactivity, IntPPC and LatPC showed considerably lower number of NAPE-PLD⫹ neurons and a less intense neuropil immunoreactivity than in the remaining cerebellar regions (Fig. 8C). Vestibular nuclei showed well-stained neurons consisting of abundant giant NAPE-PLD⫹ neurons in LVe and SpVe (Fig. 8E), and large neurons in MVeMC (Fig. 8F). MVePC also showed numerous stained cell bodies immersed in moderately NAPE-PLD⫹ neuropil (Fig. 8F). DISCUSSION Here we report the first detailed analysis of the presence and the comparative distribution of functionally relevant proteins of the endogenous cannabinoid system, namely, the two main cannabinoid receptors (CB1 and CB2), the enzymes involved in cannabinoid biosynthesis (DAGL␣, DAGL, and NAPE-PLD), and two endocannabinoid-degradating enzymes (FAAH and MAGL) in the rat cerebellum (cerebellar cortex and cerebellar nuclei) and two functionally related nuclei, the vestibular nuclei and the inferior olive. It is important to note that additional putative endocannabinoid receptors (i.e., orphan receptor GPR55, vanilloid VR1 receptor) and enzymes for biosynthesis and degradation have been proposed (Leung et al., 2006; Simon and Cravatt, 2006; Liu et al., 2007). However, their molecular characterization and their contribution to endocannabinoid physiology are still under active investigation. Our results confirm data from previous studies on the presence and localization of CB1 in the cerebellar cortex. Our study also provides new insight in relation to the localization of CB1 and CB2 receptors and FAAH, but principally in relation to the presence of MAGL, DAGL␣, 413 DAGL, and NAPE-PLD in cerebellum and functionally related nuclei that have not been described previously. Additionally, the segregated localization of CB1 and CB2 in the cerebellum suggests a complementary distribution of the two receptors associated with the specific distribution of the cannabinoid degradation and biosynthesis enzymes in the cerebellum. Because of the described variability of NAPE-PLD, CB1, and CB2 distribution with regard to the different antibodies used, we carried out careful control experiments for specificity. Thus, we have used the NAPE-PLD knockout mouse, CB1 knockout mouse, CB2 knockout mouse, and Western blot analyses as additional controls for immunohistochemistry to characterize the NAPE-PLD, CB1, and CB2 antibodies and demonstrate their antibody specificity. We observed that immunostaining was almost completely absent in CB1 knockout mouse brain. However, weak staining was found in the spinal trigeminal tract at cerebellar levels and in the cerebral peduncle at hippocampal levels (Suppl. Fig. 1). With the exception of these features, all of the staining in wild-type brain is specifically attributable to CB1 expression. We did not observed labeling in the CB2 receptor knockout mouse or NAPE-PLD receptor knockout mouse, whereas the wildtype mouse showed labelling similar to that of the rat brain (Suppl. Figs. 2, 3). CB1-immunostained bands (60 kD) were similar to those described by Egertová and Elphick (2000). CB2 immunoblotting also showed a band (55 kD) similar to that described in recent reports for rat brain (Van Sickle et al., 2005; Gong et al., 2006). The single bands observed for FAAH (63 kD) and NAPE-PLD (46 kD) were identical to those described previously (Giang and Cravatt, 1997; Okamoto et al., 2004). The DAGL␣ molecular mass (120 kD) is identical to that described for COS cells by Bisogno and collaborators (2003). Carrying out more stringent conditions for DAGL immunoblotting, we detected a prominent DAGL band (76 kD) that was similar to that described by Bisogno and collaborators (2005; 70 kD). The weaker band at 97 kD may be explained by the presence of a glycosylated form of DAGL that has yet to be defined. Analysis of MAGL immunoreactivity confirmed two bands of 37 and 35 kD, but we also observed an additional band at about 62 kD, similar to the weak band observed in Figure 2 of Dihn et al. (2002). This band can represent a post-translationally modified form of MAGL in the rat cerebellum that still has to be characterized. We carried out Western blots and immunohistochemistry in the presence of specific immunizing peptides to confirm the specificity of the labelling. The absence of labelling under these conditions indicated that the seven antibodies utilized in the present study were selective for the histological identification and discrimination of their protein expression. Distribution of CB1 and CB2 in cerebellum Our data indicated that CB1 and CB2 immunoreactivities show partial complementary localization in the cerebellar cortex. Characterization and localization of CB1immunopositive staining throughout all the cerebellar lobules was consistent with previous studies on the expression of CB1 mRNA, the detection of [3H]CP-55,940 binding sites, and the immunocytochemical mapping of CB1 in rodent brain (Herkenham et al., 1991b; Matsuda et al., 1993; Pettit et al., 1998; Egertová and Elphick, 2000; Cristino et al., 2006). We have located the majority of CB1 The Journal of Comparative Neurology 414 Fig. 8. NAPE-PLD immunoreactivity in the rat cerebellum and vestibular nuclei. A: General view of a coronal section through the cerebellum and vestibular nuclei. NAPE-PLD-immunoreactive (NAPE-PLD⫹) Purkinje somata and granular cell (arrows) are observed in the cerebellar cortex (B,D). Note the weakly stained NAPEPLD⫹ cells in the molecular layer that may correspond to basket and J. SUÁREZ ET AL. stellate cells (arrowheads). C: Low-magnification photomicrographs showing NAPE-PLD immunoreactivity throughout the cerebellar and vestibular nuclei. Note the well-labeled somata of the giant neurons in the LVe (E) and the small neurons in the MVePC (F). For abbreviations see list. Scale bars ⫽ 1 mm in A; 100 m in B,D–F; 200 m in C; 20 m in inset. Fig. 9. Photomicrographs of coronal sections through the rat inferior olive (IO), showing CB1 (A), CB2 (B), FAAH (C), MAGL (D), DAGL␣ (E), DAGL (F), and NAPE-PLD (G) immunohistochemistry. Numerous stained small cells are located in all IO subdivisions, except for CB1, CB2, and DAGL␣ immunoreactivity, whereas IO shows a denser neuropil than the surrounding areas (A,B,E). Note the restricted location of CB1⫹ neuropil in the dorsal part of the IOPr (A). H: Schematic representation of the IO subdivisions at Bregma –12.72 mm, described in the rat brain atlas of Paxinos and Watson (1998). For abbreviations see list. Scale bars ⫽ 100 m. The Journal of Comparative Neurology 416 immunoreactivity in the molecular layer of the rat cerebellar cortex, largely associated with climbing and parallel fibers that extended on the Purkinje dendrites and in clustered basket cell axons surrounding Purkinje somata, especially on their basal areas, which correspond to the initial axonal segment (Herkenham, 1995; Egertová and Elphick, 2000; Egertová et al., 2003; Cristino et al., 2006; Kawamura et al., 2006). Previous studies have reported dense [3H]CP-55,940 labelling in the molecular layer and sparse binding in the granular layer, including mutant mice deficient in Purkinje cell expression, suggesting that Purkinje cells were not the source of CB1 expression in the molecular layer (Herkenham et al., 1991a,b; Herkenham, 1995). As expected, cell bodies of the molecular layer (basket and stellate cells) expressed CB1 mRNA, but Purkinje cells did not (Mailleux and Vanderhaeghen, 1992; Matsuda et al., 1993). In agreement with these studies, we did not detect CB1 immunoreactivity in Purkinje somata and their dendritic processes (Egertová and Elphick, 2000). The present study showed an intense CB1 immunoreactivity in fibers of the molecular layer, which agrees with previous studies describing the expression of CB1 mRNA in fibers of the molecular layer and neurons of IO (Mailleux and Vanderhaeghen, 1992), which suggests the presence of CB1 immunoreactivity in climbing fibers (Pettit et al., 1998). However, by silver-enhanced immunogold, Kawamura et al. (2006) detected occasionally weak CB1 labelling in climbing fibers that terminated on the proximal Purkinje dendrites. On the other hand, our results also indicated an absence of CB1 immunoreactivity in the granular layer (as in mossy fibers), in contrast to the detection of CB1 mRNA labelling in the deep cerebellar nuclei (Mailleux and Vanderhaeghen, 1992; Matsuda et al., 1993). However, we have detected CB1⫹ immunoreactivity in fiber bundles of the superior cerebellar peduncle (possibly from the interposed and lateral cerebellar nuclei) and in fiber terminals of the red nucleus and some nuclei of the dorsal thalamus and motor cortex (data not shown). Our results revealed a distribution of CB2 immunoreactivity in part similar to that of CB1 immunoreactivity. In contrast to recent immunocytochemical studies (Ashton et al., 2006; Gong et al., 2006; Onaivi et al., 2006), CB2 immunoreactivity was not associated with Purkinje cell bodies and their dendritic processes. We have observed strong CB2 immunostaining in a number of varicose fibers (parallel fibers) in the molecular layer; most of them may be associated with granular cells, but they could also be associated with mossy fibers in the granular layer. These data match the detection of granular layer cells and neurons in brainstem and spinal cord by in situ hybridization in previous reports (Skaper et al., 1996; Van Sickle et al., 2005). The results obtained for CB1 and CB2 immunostaining indicate that CB1 and CB2 receptors are in part located in the same presynaptic structures of the cerebellar cortex, such as clustered basket cell axons (Pinceau formation), but they are also present in complementary presynaptic structures. Therefore, CB1 receptors are preferably located in climbing fibers (olivary projections), whereas CB2 receptors are preferably located in mossy fibers (spinal, pontine, and vestibular projections) and parallel fibers (cerebellar granular cells). The presynaptic localization of both cannabinoid receptors in the cerebellar cortex supports the hypothesis of endocannabinoids as retrograde messengers proposed for different brain areas, including J. SUÁREZ ET AL. cerebellum, amygdala, basal ganglia, and hippocampus (Stella et al., 1997; Rodrı́guez de Fonseca et al., 1998, 2005; Giufrida et al., 1999; Wilson and Nicoll, 2001; Wilson et al., 2001; Diana et al., 2002; Gerdeman et al., 2002; Robbe et al., 2002; Chevaleyre and Castillo, 2003). At this moment, we cannot exclude that CB2 receptors might serve as a retrograde signalling gate controlling neuronal depolarization or trophic maintenance of the synapses. In any case, the finding of CB2 receptor in the cerebellum suggests the need for reevaluating the effects of exogenous and endogenous cannabinoids on neurotransmission. Presence and distribution of cannabinoid degradation enzymes in cerebellum in relation to CB1 and CB2 receptors FAAH and MAGL are two hydrolytic enzymes that mediate the degradation of different endocannabinoids. FAAH mediates endocannabinoid degradation, including AEA, but also 2-AG, whereas MAGL was found to mediate 85% of total brain membrane 2-AG hydrolase activity (Fig. 1B; Piomelli et al., 2000; Blankman et al., 2007). As described for the synthesis of endocannabinoids, additional hydrolytic enzymes degradating anandamide and 2-AG have been recently proposed (Wei et al., 2006; Blankman et al., 2007; Muccioli et al., 2007), but their role in neural circuits is still unknown. Thus we will limit the discussion to both FAAH and MAGL. The presence of both endocannabinoid degradation enzymes in the cerebellum gives support for the existence of multiple regulatory mechanisms terminating endocannabinoid signaling. As occurs with CB1 and CB2 receptors, the specific localization of FAAH and MAGL also suggests a complementary distribution of the two enzymes in the cerebellar cortex. Consistent with previous studies of the rodent and human cerebellar cortex (Egertová et al., 1998, 2003; Romero et al., 2002; Gulyas et al., 2004), FAAH immunoreactivity was present in Purkinje somata. Additionally, we have clearly detected in sagittal cerebellar slides the characteristic dendritic tree of the Purkinje cell, including the tiniest branches, which contained intense FAAH immunostaining. These data disagree with data from Egertová et al. (2003) but agree with the dendritic staining described in the molecular layer of human and rat cerebellum (Romero et al., 2002; Gulyas et al., 2004) and observed in human cerebellar samples in our laboratory (Suarez et al., unpublished). However, we have not detected stained cells in rat cerebellar molecular layer. In contrast to previous studies in rat cerebellum (Egertová et al., 1998; Tsou et al., 1998; Gulyas et al., 2004), FAAH immunoreactivity was also evident in a small population of granular cells that was consistent with the detection of granular cells in rat cerebellum by in situ hybridization (Thomas et al., 1997) and in human and mouse cerebellum by immunohistochemistry (Romero et al., 2002; Egertová et al., 2003). All these data indicate that the localization of FAAH is quite complementary to that of CB1 and CB2 receptors in the cerebellar cortex, so Purkinje somata and their dendritic processes and a specific population of granular cells expressed FAAH, whereas theirs presynaptic structures, climbing fibers, expressed CB1 receptor, and parallel fibers and mossy fibers expressed CB2 receptor. Concerning the presence of MAGL in the cerebellum, and in contrast to the findings of Gulyas et al. (2004), we found that not only the molecular layer neuropil (possibly The Journal of Comparative Neurology ENDOCANNABINOID AND CEREBELLUM consisting of axon terminals) but also densely packed cells of the granular layer showed MAGL immunoreactivity. We have clearly distinguished two cell types according to the intensity of MAGL immunoreactivity: a dense population of moderately immunoreactive cells disposed throughout the granular layer, which may be granular cells, and lower numbers of strongly immunoreactive cells disposed near Purkinje layer, which probably are Golgi cells. Additionally, cells of the molecular layer, as well as Purkinje somata, showed moderate MAGL staining. These molecular layer cells probably represented basket cells and stellate cells. The localization of MAGL in the cerebellar cortex could partially overlap that of CB1 and CB2 receptors. Despite the difficulty in defining the specific localization of MAGL expression because of the immunoreactivity in the granular layer, the stained fibers and puncta in the molecular and granular layers could be related to mossy and climbing and/or parallel fibers; although negative Purkinje cell dendrites could be clearly seen (Gulyas et al., 2004). The location of MAGL as a presynaptic enzyme, which agreed with CB1 and CB2 receptor distribution in presynaptic structures of the cerebellar cortex, may be related to the retrograde messenger role of 2-AG, as described recently for the hippocampus, which determines basal endocannabinoid tone (Hashimotodani et al., 2007). For the present study, we have optimized the immunohistochemical protocol for MAGL antibody (kindly donated by Dr. D. Piomelli) by using a combination of immunological methods. We tested different incubations, pretreatments, and antibody dilutions in comparison with a commercial MAGL antibody (Cayman; catalog No. 100035; Suppl. Fig. 6). Both MAGL antibodies recognized the same N-terminal aa sequence and revealed the same molecular masses. Only the distinct dilutions tested for both antibodies resulted in differences in the general intensity of the MAGL immunoreactivity. In contrast, both MAGL antibodies resulted in the same molecular weight and the same immunohistochemical distribution in the rat cerebellum. Therefore, the immunohistochemical differences observed in this study and that of Gulyas et al. (2004) can only be explained by the use of different MAGL lots. Additional explanations might be considered in order to clarify the nature of this potentially nonspecific signal. Although MAGL is thought to be the major 2-AGhydrolyzing enzyme (Blankman et al., 2007), it also acts as an inactivator of other monoacylglycerol and prostaglandin glycerol esters (Dihn et al., 2002, 2004; Vila et al., 2007). Thus, MAGL is also a relevant enzyme controlling the acyl glycerol metabolism that might not be oriented only to synaptic transmission. Furthermore, a recent study has provided evidence of MAGL activity that controls 2-AG levels in microglia, not described previously (Muccioli et al., 2007). The novel MAGL activity is especially rich in mitochondrial and nuclei. The findings of Mucciolli and collaborators suggest that the cloned MAGL, which is responsible for the majority of 2-AG hydrolysis in healthy brains (Hohmann et al., 2005), does not play a major role in primary microglia. Possible explanations for this include cell-specific regulation of MAGL translation and, furthermore, the differential regulation that cytokine activation produces on the expression of MAGL and the novel MAGL in the brain. The authors observed an inverse regulation of both enzymes by 417 interferon-␥ (Witting et al., 2006). All these possibilities require further clarification but may support the evidence of a wider distribution of the enzyme in the brain. Presence and distribution of cannabinoid biosynthesis enzymes in the cerebellum in relation to CB1 and CB2 receptors We have reported here the first analysis of the presence of DAGL␣, DAGL, and NAPE-PLD in the cerebellum. The expression of these biosynthesis enzymes will determine where endocannabinoids are made and released in the cerebellum. DAGL␣ and DAGL constitute two recently identified isoforms of closely related genes correlated with 2-AG biosynthesis and release (Fig. 1B; Mechoulam et al., 1995; Sugiura et al., 1995; Piomelli et al., 2000; Bisogno et al., 2003). Additionally, Bisogno and collaborators (2003) have found coexpression of DAGL␣ and DAGL in a similar staining pattern in mouse brain. They also indicated that the expression of both isozymes changed in the developing brain from axonal tracts in the embryonic stages to dendritic fields in the adulthood. It is important to note that, even though 2-AG is considered a full cannabinoid receptor agonist, it is also an important intermediate in triacyl/diacylglycerol metabolism as well as a prominent molecule linking the cannabinoid signaling with lysophospholipids and diacycilglycerol-PKC signaling systems. Therefore, we cannot strictly consider both DAGL␣ and DAGL as pure endocannabinoid-synthesizing enzymes. Besides their obvious role on the endocannabinoid system, it is very possible that they also play other, additional physiological roles. For instance, pharmacological studies have suggested that DAGL activity is required for axonal growth and guidance in developing brain (Brittis et al., 1996; Williams et al., 2003). However, we will focus only on their potential role in the endocannabinoid system. In agreement with Bisogno and collaborators (2003), our results for the rat cerebellum showed high DAGL␣ expression in the dendritic field of the Purkinje cells, consisting of prominent tube-like structures that contained numerous varicosities on their surface. In addition, Purkinje cells expressed the highest levels of DAGL␣ mRNA but not cerebellar granular cells (Yoshida et al., 2006). However, our results indicated that the staining of DAGL was quite different from that of DAGL␣ in rat cerebellum; that is, whereas Purkinje cell dendrites strongly expressed DAGL␣, Purkinje cell bodies specifically expressed DAGL. Additionally, we have detected a small population of granular cells that was DAGL immunoreactive. The distribution of DAGL␣ mRNA and DAGL mRNA also differed in the cerebellar cortex. DAGL mRNA was highly expressed in the cerebellar granular layer (Yoshida et al., 2006). The different localizations of DAGL␣ and DAGL suggest that at least three different postsynaptic locations preferably make and release 2-AG in the cerebellar cortex: Purkinje cell dendrites postsynaptically release 2-AG by DAGL␣ and Purkinje somata and granular somata/ dendrites by DAGL. Indeed, the postsynaptic localization of DAGL␣ in Purkinje cell dendrites correlates with the presynaptic localization of CB1 receptor in climbing fibers that terminate on distal and proximal Purkinje cell dendrites and with the presynaptic location of CB2 receptor in mossy and parallel fibers that terminate on The Journal of Comparative Neurology 418 J. SUÁREZ ET AL. granular cells and Purkinje dendrites, respectively. In agreement with our results, a previous report indicated that DAGL␣ was essentially targeted by postsynaptic spines in cerebellar Purkinje cells and suggested close proximity between production sites of endocannabinoids and their receptors (Yoshida et al., 2006). The postsynaptic localization of DAGL in Purkinje somata and granular somata also agrees with the presynaptic localization of CB1 and CB2 receptors in clustered basket cell axons (Pinceau formation) and with the location of CB2 receptor in mossy fibers that surround granular somata. On the other hand, NAPE-PLD is another recently characterized cannabinoid biosynthesis enzyme that mediates the release of N-acyl ethanolamines (including AEA) from a phospholipid precursor [N-acyl-phosphatidylethanolamide (NAPE); Fig. 1B; Piomelli et al., 2000; Okamoto et al., 2004]. Again, N-acyl ethanolamides are not only endocannabinoid mediators; some of them (oleoylethanolamide, palmithylethanolamide) are also activators of other receptor types, including nuclear receptors of the peroxisome proliferatorsactivated receptor family (Fu et al., 2003). However, c-fos mapping did not reveal a substantial change in the pattern of cellular activity in the cerebellum after exogenous oleoylethanolamide administration, underscoring its contribution to cerebellar physiology (Rodriguez de Fonseca et al., 2001). Our results indicated that the staining of NAPE-PLD was quite similar to that of DAGL in the cerebellar cortex, as indicated by the fact that Purkinje somata and a small population of granular somata presented prominent NAPE-PLD immunoreactivity. Here, we have also observed weak NAPE-PLD expression in cells and fibers of the molecular layer. The appearance of these fibers in the molecular layer possibly represents the dendritic field of the Purkinje cells. So, NAPE-PLD and DAGL␣ may be coexpressed in the Purkinje cell dendritic field. Similarly to DAGL␣ and DAGL, the postsynaptic localization of NAPE-PLD in Purkinje somata and granular somata correlates with the presynaptic localization of CB1 and CB2 receptor in clustered basket cell axons (Pinceau formation) and the location of CB2 receptor in mossy fibers, whereas the postsynaptic localization of NAPE-PLD in Purkinje dendrites and molecular layer cells correlates with the presynaptic localization of CB1 receptor in climbing fibers and the location of CB2 receptor in parallel fibers. The induction of retrograde signals by the biosynthesis of endocannabinoids in Purkinje dendrites (DAGL␣⫹ and NAPE-PLD⫹) is enhanced when parallel fiber (CB2⫹) stimulation is combined with climbing fiber (CB1⫹) stimulation (Brenowitz and Regehr, 2005). Presence of the endocannabinoid system in the cerebellar nuclei, vestibular nuclei, and inferior olive The importance should be noted of the CB2 receptor in fiber terminals, which homogeneously filled all cerebellar and vestibular nuclei and suggests a Purkinje origin. In addition, IO also showed a dense network of CB2⫹ fibers that may originate principally from spinal projections. On the other hand, the presence of the CB1 receptor in fibers that coursed into the inferior cerebellar peduncles could relate to the olivary-cerebellar projections, which also correlates with the presence of climbing fibers in the cerebellar cortex (Fig. 10). Fig. 10. A: Schematic parasagittal section of a mammalian cerebellum redrawn from Cajal (1911). B: Schematic representation of the inferior olive-cerebellar and vestibulocerebellar connections and intrinsic cerebellar circuits in mammals. For abbreviations see list. These data were not consistent with previous studies showing an intense CB1 and CB2 immunoreactivities associated with neuronal somata of the cerebellar nuclei (see Fig. 7 in Pettit et al., 1998; Gong et al., 2006) and vestib- The Journal of Comparative Neurology ENDOCANNABINOID AND CEREBELLUM ular nuclei (Ashton et al., 2004; see Figs. 5F and 13A in Gong et al., 2006). However, our study agrees with the low level of [3H]CP-55,940 binding and the low intensity of CB1 mRNA signal found in the deep cerebellar nuclei, vestibular nuclei, and IO (Herkenham et al., 1991b; Matsuda et al., 1993) but also with the low levels of CB1 radiographic labelling in the human medial and lateral vestibular nucleus (Glass et al., 1997). In contrast, the presence of cannabinoid degradation and biosynthesis enzymes was prominent in cerebellar nuclei, vestibular nuclei, and IO complex. Although FAAH, MAGL, DAGL, and NAPE-PLD immunoreactivities were associated mainly with cell bodies, DAGL␣ immunoreactivity was associated exclusively with a conspicuous network of fibers and puncta in these three functionally related regions. In agreement with Bisogno and collaborators (2003), DAGL␣ expression became restricted to synaptic fields in the adult brain, possibly in postsynaptic dendrites, correlating with the postsynaptic requirement for the synthesis of endocannabinoids as a retrograde messenger (Kreitzer and Regehr, 2001; Diana et al., 2002; Bisogno et al., 2003). The presence of the proteins of the endogenous cannabinoid system in the input and output relays of the cerebellar cortex suggests a potential role for endocannabinoid-mediated plasticity in motor timing and learning mediated by these circuits (Raymond et al., 1996). Role for the endocannabinoid system in the cerebellum: new targets for the study of motor learning and the ataxias Early studies using natural and synthetic cannabinoids reported ataxia as one of the key features of the pharmacological profile of these compounds (Dewey, 1986; Patel and Hillard, 2001). The effects on ataxia are mediated by cannabinoid CB1 receptors, and apparently there are no CB2-mediated gait alterations in experimental animals (Patel and Hillard, 2001; Valenzano et al., 2005). These results indicated that, at least, motor timing and coordination require an intact endogenous cannabinoid receptor signalling system that, despite the activation of CB1 receptors, may regulate sensorimotor integration. However, the cerebellum is an additional motor learning device needed for many different forms of motor learning (Raymond et al., 1996). It has been suggested that the endogenous cannabinoid system modulates motor learning (i.e., eye blink conditioning) in the cerebellum (Kishimoto and Kano, 2006; Skosnik et al., 2007). The truth is that there is a lack of information on the role of CB1 and CB2 receptors in these learning processes involving cerebellar circuits. Most of the work so far has been performed in the cerebellar cortex, using synaptic plasticity paradigms. Recent studies have clearly established a role for the endogenous cannabinoid system in short-term and long-term forms of synaptic plasticity in the cerebellar cortex. Longterm depression, depolarization-induced suppression of inhibition, or depolarization-induced suppression of excitation is modulated by endogenous cannabinoid release (Diana and Marty, 2004; Kreitzer and Regehr, 2001; Safo and Regehr, 2005). These effects are mediated through cannabinoid CB1 receptors. However, little is known on the role of cannabinoid CB2 receptors, which, as described here, are present in clustered basket cell axons (Pinceau formations) and other relevant relays of the cerebellar 419 circuitry. Whether they affect postsynaptic endocannabinoid production, downstream intracellular signalling, or survival/remodelling processes still requires further investigation. This is relevant not only for motor learning but also for the neuroadaptions associated with cerebellar insults or chronic degenerative disorders such as the ataxias. Moreover, we lack crucial information on the role of the endogenous cannabinoid system in deep cerebellar nuclei and the IO complex. Because of the crucial role of these structures in motor timing and learning, especially in sensorymotor integration and conditioning, it is possible to anticipate a role for cannabinoid receptors in modulating the input and output information streams of the cerebellar cortex, beyond the Purkinje cell (as an example, it is worth remembering that the IO is the sole source for an entire afferent system to the cerebellum, the climbing fibers). More research is thus needed to establish this hypothesis by analyzing the effects of cannabinoid CB1 and CB2 receptors as well as FAAH and MAGL inhibitors in simple learning paradigms, such as the vestibuloocular reflex and the eyelid conditioning responses. Such studies may also help to establish the potential pharmacological utility of cannabinoids in cerebellar disorders. ACKNOWLEDGMENTS The authors are indebted to Dr. Daniele Piomellli for kindly providing MAGL antibody. LITERATURE CITED Ashton JC, Zheng Y, Liu P, Darlington CL, Smith PF. 2004. Immunohistochemical characterisation and localisation of cannabinoid CB1 receptor protein in the rat vestibular nucleus complex and the effects of unilateral vestibular deafferentation. Brain Res 1021:264 –271. Ashton JC, Friberg D, Darlington CL, Smith PF. 2006. Expression of the cannabinoid CB2 receptor in the rat cerebellum: an immunohistochemical study. Neurosci Lett 396:113–116. Benito C, Kim WK, Chavarria I, Hillard CJ, Mackie K, Tolon RM, Williams K, Romero J. 2005. A glial endogenous cannabinoid system is upregulated in the brains of macaques with simian immunodeficiency virusinduced encephalitis. J Neurosci 25:2530 –2536. Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. 2003. Cloning of the first sn1-dag lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol 163:463– 468. Bisogno T, Ligresti A, Di Marzo V. 2005. The endocannabinoid signalling system: biochemical aspects. Pharmacol Biochem Behav 81:224 –238. Blankman JL, Simon GM, Cravatt BF. 2007. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol 14:1347–1356. Brenowitz SD, Regehr WG. 2005. Associative short-term synaptic plasticity mediated by endocannabinoids. Neuron 45:419 –31. Brittis PA, Silver J, Walsh FS, Doherty P. 1996. Fibroblast growth factor receptor function is required for the orderly projection of ganglion cell axons in the developing mammalian retina. Mol Cell Neurosci 8:120 – 128. Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer AM, Felder CC, Glass M, Zimmer A. 2000. Immunomodulation by cannabinoids is absent in mice deficient for the CB2 cannabinoid receptor. Eur J Pharmacol 396:141–149. Chevaleyre V, Castillo PE. 2003. Heterosynaptic LTD of hippocampal gabaergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron 38:461– 472. Cravatt BF, Prospero-Garcia O, Siuzdak G, Gilula NB, Henriksen SJ, Boger DL, Lerner RA. 1995. Chemical characterization of a family of brain lipids that induce sleep. Science 268:1506 –1509. Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Guilula NB. The Journal of Comparative Neurology 420 1996. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83– 87. Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. 2001. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A 98:9371–9376. Cristino L, De Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo D. 2006. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptor in the mouse brain. Neuroscience 139:1405–1415. Dewey WL. 1986. Cannabinoid pharmacology. Pharmacol Rev 38:151–178. Diana MA, Marty A. 2004. Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE). Br J Pharmacol 142:9 –19. Diana MA, Levenes C, Mackie K, Marty A. 2002. Short-term retrograde inhibition of GABAergic synaptic currents in rat Purkinje cells is mediated by endogenous cannabinoids. J Neurosci 22:200 –208. Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. 2002. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A 99:10819 – 10824. Dinh TP, Kathuria S, Piomelli D. 2004. RNA interference suggests a primary role for monoacylglycerol lipase in the degradation of the endocannabinoid 2 arachidonoylglycerol. Mol Pharmacol 66:1260 – 1264. Egertová M, Elphick MR. 2000. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB1. J Comp Neurol 422:159 –171. Egertová M, Giang DK, Cravatt BF, Elphick MR. 1998. A new perspective on cannabinoid signalling: complementary localization of fatty acid amide hydrolase and the CB1 receptor in rat brain. Proc R Soc Lond B Biol Sci 265:2081–2085. Egertová M, Cravatt BF, Elphick MR. 2003. Comparative analysis of fatty acid amide hydrolase and CB(1) cannabinoid receptor expression in the mouse brain: evidence of a widespread role for fatty acid amide hydrolase in regulation of endocannabinoid signaling. Neuroscience 119: 481– 496. Ferrer B, Bermudez-Silva FJ, Bilbao A, Alvarez-Jaimes L, Sanchez-Vera I, Giuffrida A, Serrano A, Baixeras E, Khaturia S, Navarro M, Parsons LH, Piomelli D, Rodriguez de Fonseca F. 2007. Regulation of brain anandamide by acute administration of ethanol. Biochem J 404:97– 104. Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, Rosengarth A, Luecke H, Di Giacomo B, Tarzia G, Piomelli D. 2003. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature 425:90 –93. Gerdeman GL, Ronesi J, Lovinger DM. 2002. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci 5:446 – 451. Giang DK, Cravatt BF. 1997. Molecular characterization of human and mouse fatty acid amide hydrolases. Proc Natl Acad Sci U S A 94:2238 – 2242. Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. 1999. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci 2:358 –363. Glass M, Dragunow M, Faull RL. 1997. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 77:299 –318. Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. 2006. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res 1071:10 –23. Goparaju SK, Ueda N, Yamaguchi H, Yamamoto S. 1998. Anandamide amidohydrolase reacting with 2-arachidonoylglycerol, another cannabinoid receptor ligand. FEBS Lett 422:69 –73. Gulyas AI, Cravatt BF, Bracey MH, Dihn TP, Piomelli D, Boscia F, Freund TF. 2004. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci 20:441– 458. Hashimotodani Y, Ohno-Shosaku T, Kano M. 2007. Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid signaling in the hippocampus. J Neurosci 27:1211–1219. Herkenham M. 1995. Localization of cannabinoid receptors in brain and J. SUÁREZ ET AL. periphery. In: Pertwee R, editor. Cannabinoid receptors. London: Academic Press. p 145–166. Herkenham M, Groen BGS, Lynn AB, deCosta BR, Richfield EK. 1991a. Neuronal localization of cannabinoid receptors and second messengers in mutant mouse cerebellum. Brain Res 552:301–310. Herkenham M, Lynn A, Johnson MR, Melvin LS, de Costa BR, Rice KC. 1991b. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 11:563–583. Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. 2005. An endocannabinoid mechanism for stress-induced analgesia. Nature 435:1108 –1112. Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. 2006. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci 26:2991–3001. Kishimoto Y, Kano M. 2006. Endogenous cannabinoid signaling through the CB1 receptor is essential for cerebellum-dependent discrete motor learning. J Neurosci 26:8829 – 8837. Kreitzer AC, Regehr WG. 2001. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci 21:1–5RC174. Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Böhme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. 1999. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 283:401– 404. Leung D, Saghatelian A, Simon GM, Cravatt BF. 2006. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry 45:4720 – 4726. Liu J, Wang L, Harvey-White J, Huang BX, Kim HY, Luquet S, Palmiter RD, Krystal G, Rai R, Mahadevan A, Razdan RK, Kunos G. 2007. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology [June 6 E-pub ahead of print]. Lu Q, Straiker A, Lu Q, Maguire G. 2000. Expression of CB2 cannabinoid receptor mRNA in adult rat retina. Vis Neurosci 17:91–95. Mailleux P, Vanderhaeghen JJ. 1992. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience 48:655– 668. Matsuda LA, Bonner TI, Lolait SJ. 1993. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol 327:535–550. Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. 1995. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50:83–90. Muccioli GG, Xu C, Odah E, Cudaback E, Cisneros JA, Lambert DM, Lopez Rodriguez ML, Bajjalieh S, Stella N. 2007. Identification of a novel endocannabinoid-hydrolyzing enzyme expressed by microglial cells. J Neurosci 2007 27:2883–2889. Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. 2004. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem 279:5298 –305. Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, Brusco A, Akinshola BE, Liu QR, Hope B, Iwasaki S, Arinami T, Teasenfitz L, Uhl GR. 2006. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci 1074:514 –536. Patel S, Hillard CJ. 2001. Cannabinoid CB(1) receptor agonists produce cerebellar dysfunction in mice. J Pharmacol Exp Ther 297:629 – 637. Paxinos G, Watson C. 1998. The rat brain in stereotaxic coordinates, 24th ed. London: Academic Press. Pazos MR, Nunez E, Benito C, Tolon RM, Romero J. 2004. Role of the endocannabinoid system in Alzheimer’s disease: new perspectives. Life Sci 75:1907–1915. Pettit DAD, Harrison MP, Olson JM, Spencer RF, Cabral GA. 1998. Immunohistochemical localization of the neural cannabinoid receptor in rat brain. J Neurosci Methods 51:391– 402. Piomelli D, Giuffrida A, Calignano A, Rodrı́guez de Fonseca F. 2000. The endocannabinoid system as a target for therapeutic drugs. Trends Pharmacol Sci 21:218 –224. Raymond JL, Lisberger SG, Mauk MD. 1996. The cerebellum: a neuronal learning machine? Science 272:1126 –1131. The Journal of Comparative Neurology ENDOCANNABINOID AND CEREBELLUM Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. 2002. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A 99:8384 – 8348. Rodrı́guez de Fonseca F, Del Arco I, Martin-Calderon JL, Gorriti MA, Navarro M. 1998. Role of the endogenous cannabinoid system in the regulation of motor activity. Neurobiol Dis 5:483–501. Rodriguez de Fonseca F, Navarro M, Gomez R, Escuredo L, Nava F, Fu J, Murillo-Rodriguez E, Giuffrida A, LoVerme J, Gaetani S, Kathuria S, Gall C, Piomelli D. 2001. An anorexic lipid mediator regulated by feeding. Nature 414:209 –212. Rodrı́guez de Fonseca F, Del Arco I, Bermúdez-Silva FJ, Bilbao A, Cippitelli A, Navarro M. 2005. The endocannabinoid system: physiology and pharmacology. Alcohol Alcoholism 40:2–14. Romero J, Hillard CJ, Calero M, Rábano A. 2002. Fatty acid amide hydrolase localization in the human central nervous system: an immunohistochemical study. Brain Res Mol Brain Res 100:85–93. Safo PK, Regehr WG. 2005. Endocannabinoids control the induction of cerebellar LTD. Neuron 48:647– 659. Sheng WS, Hu S, Min X, Cabral GY, Lokensgard JR, Peterson PK. 2005. Synthetic cannabinoid WIN55212-2 inhibits generation of inflammatory mediators by IL-IB-stimulated human astrocytes. Glia 49:211– 219. Skaper SD, Buriani A, Dal Toso R, Petrelli L, Romanello S, Facci L, Leon A. 1996. The ALIAmide palmitoylethanolamide and cannabinoids, but not anandamide, are protective in a delayed postglutamate paradigm of excitotoxic death in cerebral granule neurons. Proc Natl Acad Sci U S A 93:3984 –3989. Skosnik PD, Edwards CR, O’Donnell BF, Steffen A, Steinmetz JE, Hetrick WP. 2007. Cannabis use disrupts eyeblink conditioning: evidence for cannabinoid modulation of cerebellar-dependent learning. Neuropsychopharmacology [July 18 E-pub ahead of print]. Simon GM, Cravatt BF. 2006. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/ beta-hydrolase 4 in this pathway. J Biol Chem 281:26465–26472. Stella N, Schweitzer P, Piomelli D. 1997. A second endogenous cannabinoid that modulates long-term potentiation. Nature 388:773–778. Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 1995. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215:89 –97. Thomas EA, Cravatt BF, Danielson PE, Gilula NB, Sutcliffe JG. 1997. Fatty acid amide hydrolase, the degradative enzyme for anandamide and oleamide, has selective distribution in neurons within the rat central nervous system. J Neurosci Res 50:1047–1052. Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. 1998. Immu- 421 nohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83:393– 411. Valenzano KJ, Tafesse L, Lee G, Harrison JE, Boulet JM, Gottshall SL, Mark L, Pearson MS, Miller W, Shan S, Rabadi L, Rotshteyn Y, Chaffer SM, Turchin PI, Elsemore DA, Toth M, Koetzner L, Whiteside GT. 2005. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology 48:658 – 672. Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. 2005. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310:329 –332. Vila A, Rosengarth A, Piomelli D, Cravatt B, Marnett LJ. 2007. Hydrolysis of prostaglandin glycerol esters by the endocannabinoid-hydrolyzing enzymes, monoacylglycerol lipase and fatty acid amide hydrolase. Biochemistry 46:9578 –9585. Wager-Miller J, Westenbroek R, Mackie K. 2002. Dimerization of G protein-coupled receptors: CB1 cannabinoid receptors as an example. Chem Phys Lipids 121:83– 89. Wei BQ, Mikkelsen TS, McKinney MK, Lander ES, Cravatt BF. 2006. A second fatty acid amide hydrolase with variable distribution among placental mammals. J Biol Chem. 281:36569 –36578. Williams EJ, Walsh FS, Doherty P. 2003. The FGF receptor uses the endocannabinoid signaling system to couple to an axonal growth response. J Cell Biol 160:481– 486. Wilson RI, Nicoll RA. 2001. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410:588 –592. Wilson RI, Nicoll RA. 2002. Endocannabinoid signaling in the brain. Science 296:678 – 682. Wilson RI, Kunos G, Nicoll RA. 2001. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron 31:453– 462. Witting A, Chen L, Cudaback E, Straiker A, Walter L, Rickman B, Moller T, Brosnan C, Stella N. 2006. Experimental autoimmune encephalomyeli-tis disrupt endocannabinoid-mediated neuroprotection. Proc Natl Acad Sci U S A 103:6362– 6367. Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. 2006. Localization of diacylglycerol lipase-␣ around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci 26:4740 – 4751. Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O’Donnell D. 2003. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci 17:2750 –2754.