* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Plastid genes transcribed by the nucleus
Genomic imprinting wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Chloroplast DNA wikipedia , lookup
Genome (book) wikipedia , lookup
Polyadenylation wikipedia , lookup
Biology and consumer behaviour wikipedia , lookup
Designer baby wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Non-coding DNA wikipedia , lookup
Genetically modified crops wikipedia , lookup
Ridge (biology) wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Genome evolution wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Minimal genome wikipedia , lookup
Microevolution wikipedia , lookup
RNA interference wikipedia , lookup
RNA silencing wikipedia , lookup
History of RNA biology wikipedia , lookup
Epitranscriptome wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Long non-coding RNA wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Short interspersed nuclear elements (SINEs) wikipedia , lookup
Gene expression profiling wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Non-coding RNA wikipedia , lookup
Epigenetics of human development wikipedia , lookup
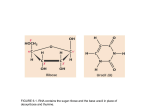
Journal of Experimental Botany, Vol. 53, No. 379, pp. 2341±2349, December 2002 DOI: 10.1093/jxb/erf108 Plastid genes transcribed by the nucleus-encoded plastid RNA polymerase show increased transcript accumulation in transgenic plants expressing a chloroplast-localized phage T7 RNA polymerase Alan M. Magee and Tony A. Kavanagh1 Plant Molecular Biology Laboratory, Smur®t Institute of Genetics, Trinity College, Dublin 2, Ireland Received 31 May 2002; Accepted 2 August 2002 Abstract A gene fusion encoding a plastid-targeted bacteriophage T7 RNA polymerase (T7RNAP) under the transcriptional control of the light-regulated promoter and the plastid-targeting signals of a ribulose-bisphosphate carboxylase/oxygenase (Rubisco) small-subunit (SSU) gene was introduced into the nuclear genome of Nicotiana tabacum (tobacco). Immunoblot analysis, in vitro transcription assays and protease treatment of isolated chloroplasts revealed that T7RNAP activity was localized within chloroplasts. RNA gel blot analyses showed a substantial increase in transcript abundance for several plastid genes that are normally transcribed by the nucleus-encoded plastid RNA polymerase (NEP) including rpoC1, rpl33, rps18, rps12, and clpP. By contrast, no signi®cant changes were observed in the levels of psbD, 16SrDNA, and ndhA transcripts. These results suggest a possible direct or indirect T7RNAP-mediated enhancement of transcription of a subset of plastid genes that contain NEP promoters. Despite these alterations in plastid transcript levels, the plants showed no visible abberant phenotype. Key words: Chloroplast, NEP, PEP, plastid transcripts, T7 RNA polymerase. Introduction The plastid genome of higher plants contains approximately 120 genes that primarily encode components of the plastid genetic system and the photosynthetic apparatus. 1 Most plastid genes are organized into operons that are transcribed by at least two distinct RNA polymerase activities: the plastid-encoded plastid RNA polymerase (PEP) and NEP. PEP is a multisubunit eubacterial-like RNA polymerase whose core subunits are encoded by the plastid rpoA, rpoB, rpoC1, and rpoC2 genes. It is responsible for the transcription of the photosynthesis genes and is the predominant transcriptional activity found in mature chloroplasts. PEP recognizes E. coli s70-type promoters containing TTGACA (±35) and TATAAT (±10) consensus elements, which are found upstream of most plastid transcription units. The recent identi®cation of nuclear genes encoding plastid-targeted sigma-like factors (SLFs) in Arabidopsis thaliana (Tanaka et al., 1997; Isono et al., 1997; Kanamura et al., 1999) and in a number of other higher plants (reviewed in Allison, 2000) suggests that promoter selection by PEP is controlled in a manner similar to that of the RNA polymerase of Escherichia coli. The differential expression patterns of genes coding for SLFs in tobacco (Oikawa et al., 2000) and maize (Sushmita et al., 2000) is indicative of their importance in regulating PEP-mediated gene expression. The existence of NEP was established by the detection of transcription from non-PEP-type promoters in DrpoB plants (non-photosynthetic plants containing a plastid rpoB deletion) (Allison et al., 1996), non-photosynthetic tobacco suspension cultures (Vera and Sugiura, 1995) and the non-green tissues of the barley albostrians (Hess et al., 1993) and maize iojap (Han et al., 1993) mutants, all of which lack PEP activity. The cloning and sequencing of nuclear genes from A. thaliana (Hedtke et al., 1997), maize (Chang et al., 1999), and wheat (Ikeda and Gray, 1999) To whom correspondence should be addressed. Fax: +353 1 6798558. E-mail: [email protected] ã Society for Experimental Biology 2002 2342 Magee and Kavanagh encoding a plastid-targeted RNA polymerase has established that NEP is a single subunit enzyme sharing homology with the RNA polymerases of phage T3 and T7. Signi®cant conservation of functional domains and important catalytic residues between T7RNAP and the maize NEP has been described (Chang et al., 1999). Moreover, NEP but not PEP has been shown to be capable of initiating transcription in vitro from a phage T7 promoter fragment and antibodies that recognize and inhibit NEP activity also recognize and inhibit T7RNAP (Bligny et al., 2000). Transcriptional patterns and transcript mapping in PEP-de®cient plants has revealed that plastid genes and operons can be assigned to three classes, those that contain (1) PEP promoters only, (2) both PEP and NEP promoters and (3) NEP promoters only. These studies have revealed that the photosystem genes are transcribed from PEP promoters and that genetic system genes are transcribed from both PEP and NEP promoters. This has led to the proposal that NEP is primarily responsible for the initiation and maintenance of the genetic system during plastid development (and in non-green tissues), and is largely replaced by PEP in mature chloroplasts. NEP, nevertheless, plays a crucial role in chloroplasts because it is exclusively responsible for transcription of the rpoB operon (Hess et al., 1993; Silhavy and Maliga, 1998) and accD (Hajdukiewicz et al., 1997). Thus, NEP ultimately controls the production of PEP and, in consequence, other components required for normal plastid functions. Sequence alignments around NEP transcription initiation sites (Hajdukiewicz et al., 1997; HuÈbschmann and Borner, 1998) have identi®ed conserved elements in NEP promoters and their functionality has been con®rmed by assaying NEP promoter mutants in NEP-containing in vitro transcription reactions (Liere and Maliga, 1999; Kapoor and Sugiura, 1999). NEP promoters consist of a conserved sequence block of about 15 nucleotides that contains a critical YRT motif located just upstream of the transcription initiation site. In addition, many NEP promoters contain a second conserved element of about 10 nucleotides located 10±20 nucleotides upstream of the YRT box. Non-consensus (exceptional) NEP promoters have also been identi®ed, for example, associated with clpP in tobacco (Sriraman et al., 1998) and the ribosomal RNA operon (rrn) in spinach (reviewed in Lerbs-Mache, 2000). It is possible that these promoters require speci®c activating factors or they may be recognized by a second NEP activity. Evidence for the existence of an additional NEP activity derives from the isolation of functionally distinct NEP activities in spinach chloroplasts (Bligny et al., 2000) and the identi®cation of two genes coding for plastidtargeted NEP-like isozymes in A. thaliana (Hedtke et al., 2000). There is increasing biotechnological interest in developing strategies to direct high-level speci®c expression of transgenes within plastids. One such strategy involves placing the transgene under the transcriptional control of a non-plastid (e.g. phage) promoter and its cognate RNA polymerase. For example, constitutive expression of a nucleus-encoded, plastid-targeted T7RNAP has been reported to direct the speci®c expression of a T7 promoter±GUS transgene located in the tobacco plastid genome (McBride et al., 1994). However, it was not determined whether the accumulation of T7RNAP, a NEPlike RNA polymerase, within plastids affected transcription of the native plastid genes. Given the similarities between NEP and T7RNAP (discussed above), the possibility of transcriptional interference as a direct or indirect consequence of the presence of T7RNAP within plastids merits investigation. In the present report, the aim was to determine whether the accumulation of T7RNAP in chloroplasts affected wild-type patterns of plastid gene transcription. Transgenic tobacco plants were generated which expressed a chimeric gene coding for a plastid-targeted T7RNAP under the control of a light-regulated promoter (hereafter referred to as SSU-T7 plants). RNA transcript levels were then compared for a number of genetic system genes (rpoC1, 16S rDNA, rpl33, rps18, and rps12), a photosystem gene (psbD) and the gene encoding the proteolytic subunit of the Clp ATP-dependent protease (clpP) in wild-type and SSUT7 plants. Fully expanded leaves of SSU-T7 plants contained increased levels of RNA transcripts for rpoC1, rpl33, rps18, rps12, and clpP when compared with wild-type plants whereas transcript levels for psbD, 16S rDNA and ndhA were largely unchanged. All of the genes showing increased transcript levels contain NEP promoters, suggesting that increased accumulation of transcripts was caused either by T7RNAP-mediated transcription from NEP promoters or by increased NEP activity in SSU-T7 plants. Materials and methods Plasmid construction The Agrobacterium tumefaciens binary vector pBinTGS25 which contains the SSU-T7 RNA polymerase translational fusion was constructed as follows. The SSU promoter and adjacent sequences encoding the transit peptide and 25 amino acids of the mature SSU (Mazur and Chui, 1985) was excised as a HindIII/MfeI restriction fragment from plasmid pCAR2 (unpublished plasmid) and ligated into the HindIII/EcoRI digested plasmid pAR3132 (Dunn et al., 1988). This resulted in the creation of an in-frame fusion of codon 25 of mature SSU with codon 11 of the T7RNAP gene. This gene fusion (ST25) was excised as a HindIII/BamHI fragment and ligated upstream of the nopaline synthase (nos) terminator region (Jefferson et al., 1987) in plasmid pRok2, a derivative of plasmid pBin19 (Bevan, 1984). Plastid transcripts in tobacco 2343 A GUS expression cassette was cloned downstream of the ST25 gene fusion in pBinTGS25 and was constructed as follows. The CaMV 35S promoter (Odell et al., 1985) was excised by EcoRI/ BamHI digestion from plasmid pRok1 (Baulcombe et al., 1986) and was ligated with a BamHI/EcoRI restriction fragment containing the GUS gene (uidA locus of E. coli) and the nos terminator. Both fragments were ligated into the EcoRI site immediately downstream of ST25 to give pBinTGS25. Transformation of tobacco Nuclear transformation was carried out by cocultivation of wounded tobacco leaf tissue with A. tumefaciens LBA4404 and the regeneration of kanamycin-resistant plants as described by Horsch et al. (1985). RNA gel-blot analyses Total leaf RNA was prepared from fully expanded leaves using the method described by Kristel et al. (1996) except that liquid nitrogen was used for sample homogenization. RNA was electrophoresed on 1% agarose±formaldehyde gels and blotted onto Hybond-N membranes (Amersham) by capillary transfer (Sambrook et al., 1989). Double-stranded DNA probes were prepared by random-primed 32Plabelling of PCR-generated fragments. The sequence of the PCR primers and their positions in tobacco plastid DNA (Shinozaki et al., 1986) are shown in Table 1. RNA gel-blot hybridizations with 32P-labelled probes and subsequent membrane washing was carried out as described in Maniatis et al. (1982). Signal detection was carried out by autoradiography using X-ray ®lm (AGFA) and Hyperscreen intensifying screens (Amersham). In vitro T7RNAP transcription assay T7RNAP activity was assayed by the incorporation of [a-32P] UTP (Amersham) into phage T7 DNA transcripts from 5 ml of total leaf or puri®ed chloroplast proteins. Reactions were carried out in 30 ml ®nal volume transcription assay mixture (40 mM TRIS±HCl, pH 8, 6 mM MgCl2, 10 mM dithiothreitol, 2 mM spermidine. 0.2 mM A/C/GTP, 0.1 mM UTP, and 1 mCi of [a32 P] UTP) that was incubated at 37 °C for 30 min. Bovine serum albumin (0.5 mg ml±1) and tRNA (1 mg ml±1) was added and transcripts were precipitated by the addition of trichloroacetic acid (10%) and incubation on ice for 30 min. Transcripts were recovered by microfugation and count per minute (cpm) values were determined for the radioactive pellet using the Packard Tri-CarbR 1500 liquid scintillation analyser. Protein preparation and quantitation Total soluble leaf and root proteins were prepared by homogenization of tissue in 50 mM sodium phosphate, pH 7, 10 mM bmercaptoethanol, and 1% Triton X-100 with a mortar and pestle in a 2:1 buffer to tissue ratio. Following a brief centrifugation protein concentration was determined using the Bradford assay (Bradford, 1976). Immunoblotting Proteins were resolved in 6.75% denaturing sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS±PAGE) as described by Sambrook et al. (1989). Protein samples were prepared for SDS±PAGE as described by Laemmli (1970). Protein was transferred to nitrocellulose membranes (Schleicher & Schuell) in a BioRad TransblotÔ apparatus at 120 mA overnight. The membrane was blocked with non-fat dried milk and incubated with polyclonal anti-T7RNAP antibodies. Signal detection was carried out by chemiluminescence using 5-bromo-4-chloro-3-indolyl phosphate (40 mg ml±1) and nitro blue tetrazolium (80 mg ml±1). Chloroplast isolation Green leaves (2±3 g) were gently disrupted by a few short bursts using a Moulinex blender in ice-cold GR buffer as described by Bartlett et al. (1982). The homogenate was ®ltered through two layers of miracloth and centrifuged at 1500 rpm for 10 min at 4 °C. Crude chloroplast pellets were resuspended in 2 ml of GR buffer and intact chloroplasts were isolated by sedimentation in Percoll density gradients (Bartlett et al., 1982). Intact chloroplasts were washed free of Percoll and resuspended in 330 mM sorbitol, 50 mM HEPES, pH 7.5 (SH buffer) at a chlorophyll concentration of 0.5 mg ml±1. Chlorophyll concentration was determined as described by Arnon (1949). Protease protection experiments Intact chloroplasts were incubated on ice for 30 min with thermolysin protease Type X (Sigma) at a concentration of 250 mg thermolysin mg±1 of chlorophyll. Intact chloroplasts were lysed by the addition of Triton X-100 to 0.1%. Thermolysin activity was inhibited by the addition of EGTA (ethylene glycol-bis(baminoethyl ether) N,N,N¢,N¢-tetraacetic acid (Sigma)) to a ®nal concentration of 20 mM. Table 1. The sequence of the PCR primers and their positions in tobacco plastid DNA (Shinozaki et al., 1986) C denotes that the identifying nucleotide is complementary to the nucleotide at that position in plastid DNA. Gene Plastid genome location Primer sequence rpoC1 22028 22549(C) 70339 70998(C) 100088/142424(C) 100647(C)/141649 73086 3665(C) 34463 35072(C) 103121/139430(C) 103750(C)/138779 122101 122800(C) GGTACATGAACAGCCATTTG ATTAGTACAAGAAGCCGTGG TGTGTCTTACCCTTTCAAGG AATGAACTCCGGGAAGGTAGAGTAG GGTGGATCTCGAAAGATATG GTAAAGGATCGTCAACAAGG GTTAAGAACTCTCCGGCATG CGCATGTACGGTTCCTAAGG TGACTATAGCCCTTGGTAAG CCGGCAACTCCCATCATATG GCAATGCCGCGTGGAGGTAG GTGTCCGCGTGCCCGAAGGC CGCTTCCACTATATCAACTG ATCAAGAAGTACTCCCCATG rpl33/rps18 3¢-rps12 clpP psbD 16S rDNA ndhA 2344 Magee and Kavanagh Fig. 1. Structure of the ST25 and linked GUS transgenes. (A) Diagram showing the ST25 (SSU-T7RNAP gene fusion) and the linked GUS expression cassettes in the binary transformation vector pBinTGS25. The ST25 transgene was placed under the transcriptional control of the light-inducible SSU promoter while GUS expression was under the control of the constitutive CaMV 35S promoter. Both cassettes contain the nos transcriptional terminator. (B) Nucleotide and deduced amino acid sequence at the SSU±T7RNAP junction. Codon 25 of mature SSU sequence was fused in-frame with codon 11 of T7RNAP. Results Generation and analysis of transgenic tobacco lines expressing a plastid-targeted T7RNAP (SSU-T7 plants) In order to generate transgenic plants with the potential to express T7 promoter-controlled transgenes located on the chloroplast genome, N. tabacum was transformed with a nuclear transgene coding for a plastidtargeted T7RNAP. To achieve this, a binary vector, pBinTGS25, was constructed which contained a gene fusion (ST25) consisting of the SSU promoter and adjacent sequences encoding the transit peptide and the N-terminal 25 amino acids of the mature SSU protein, fused in-frame with the gene coding for T7RNAP (Fig. 1). The SSU promoter was chosen to control ST25 expression in order speci®cally to direct highlevel expression in green tissues (Jefferson et al., 1987) and, therefore, avoid the potentially deleterious effects of constitutive expression of T7RNAP during embryo and seed development. The SSU transit peptide has been shown to confer ef®cient accumulation of fusion proteins in plastids (Comai et al., 1988). In addition, pBinTGS25 contained the GUS gene cloned downstream of ST25, and placed under the control of the constitutive CaMV 35S promoter and the nos terminator region. Kanamycin-resistant SSU-T7 plant lines were regenerated following A. tumefaciens-mediated transformation. GUS expression was used as a simple biochemical marker of transformation and was used to follow the segregation of ST25 in the progeny of transgenic plants (results not shown). Plant lines segregating 3:1 for the Fig. 2. Expression and chloroplast localization of the ST25 fusion protein in SSU-T7 plants. (A) Detection of ST25 in total soluble leaf protein extracts from SSU-T7 plant lines (Nos 19, 20, 35, 33, 29, 27, 23, and 15) by immunoblotting with anti-T7 RNA polymerase antibodies. Each lane contained approximately 50 mg of total leaf protein. T7 denotes 10 units of puri®ed commercial T7 RNA polymerase. Wt denotes wild-type plants. (B) Detection of ST25 in leaf tissue (L), but not in roots (R), of an SSU-T7 plant. Each lane contained approximately 20 mg of total leaf and root protein, respectively. (C) Chloroplast localization of the ST25 fusion protein. ST25 detection in total soluble leaf protein (L), and isolated chloroplasts that were either untreated (UN), or treated with thermolysin (T), or lysed with Triton X-100 followed by thermolysin treatment (TT) (upper panel). The level of Rubisco-LSU in each sample was determined by staining a duplicate protein gel with Coomassie Blue (bottom panel). kanamycin resistance gene (and presumably the linked ST25 gene) were identi®ed by germinating seeds from selfpollinated primary transformants in the presence of kanamycin. The ST25 fusion protein shows leaf-speci®c expression and accumulation in chloroplasts ST25 expression was detected by immunoblot analysis of total leaf protein preparations from SSU-T7 plants using anti-T7RNAP antibodies (Fig. 2A). ST25 was detected as a single band with the expected molecular mass of about 100 kDa which was absent in wild-type plants. As expected for nuclear transformed plants, the levels of ST25 varied between SSU-T7 lines, with some plants containing barely detectable levels while others contained levels several-fold higher than the level corresponding to 10 units of puri®ed commercial T7RNAP that was loaded as a positive control. Leaf-speci®c expression was demonstrated by showing Plastid transcripts in tobacco 2345 ef®ciency with which ST25 is targeted into chloroplasts was obtained by comparing the levels of ST25 in a total leaf protein extract and in intact chloroplasts. Since each of the protein samples analysed contained similar levels of ST25 and similar levels of the Rubisco large subunit (LSU), a marker of chloroplast stromal protein content, it can be concluded that ST25 is ef®ciently targeted to chloroplasts in SSU-T7 plants (Fig. 2C). Plants expressing the ST25 fusion protein possess a very stable T7RNAP activity Fig. 3. Expression and stability of T7RNAP activity in SSU-T7 plants. (A) T7RNAP activity was determined in units mg±1 of total soluble leaf protein in a single plant from each of seven SSU-T7 transgenic lines (Nos 15, 23, 24, 28, 29, 33, and 35). Wt denotes wildtype non-transgenic plant. One unit of T7RNAP is the enzyme activity that incorporates 1 nmol of UMP into acid-precipitable RNA products in 60 min at 37 °C. Unit values for T7RNAP activity in SSU-T7 plants were derived by comparison with a puri®ed commercial enzyme preparation. (B) Stability of T7RNAP activity in vivo. SSU-T7 seedlings were placed in total darkness and T7RNAP activity was determined at ®ve time points: days 0, 1, 5, 9, and 16 following exposure to total darkness. The activity present at day 0 was set at 100%. that ST25 was not detected in a total root protein extract from a high expressing line (Fig. 2B). In order to determine the subcellular location of ST25 in the leaves of SSU-T7 plants, puri®ed intact chloroplasts were isolated, treated with the protease thermolysin which degrades externally-associated proteins and analysed for the presence of ST25 by immunoblotting (Fig. 2C). ST25 was shown to be protected from proteolysis in thermolysin-treated intact chloroplasts, but was degraded when chloroplast membranes were disrupted with Triton X-100 prior to the thermolysin treatment. An estimate of the Following translocation into chloroplasts, the ST25 fusion protein is predicted to comprise T7RNAP with an N-terminal extension consisting of the ®rst 25 amino acids of the mature form of SSU. For this reason, it was necessary to demonstrate that ST25 retained enzymatic activity. In order to test this, total leaf protein extracts from a number of SSU-T7 plants were analysed for T7RNAP activity using an in vitro transcription system and phage T7 DNA as template. The template speci®city of the in vitro transcription system was demonstrated by showing that transcription was observed only when phage T7 DNA was included in the in vitro transcription assays; transcription was not observed either in the absence of T7 DNA or when phage lambda DNA was used as a substitute template (results not shown). Using this system, T7RNAP activity was shown to be present in the leaves of seven SSU-T7 plant lines whereas wild-type plant leaves contained negligible activity (Fig. 3A). The activity values for the group of plants analysed ranged between 0.4 and 1.4 units mg±1 of total leaf protein. In addition, it was shown that the T7RNAP activity, like the ST25 fusion protein, was localized within chloroplasts by performing in vitro transcription assays on isolated intact chloroplasts that had been treated with thermolysin (data not shown). Transcription directed by the SSU promoter is rapidly down-regulated when plants are placed in continuous darkness (Giuliano et al., 1988; Fritz et al., 1991). On the assumption that this would prevent further ST25 transgene expression, an estimate of the stability of the ST25 fusion protein in vivo can be obtained by transferring SSU-T7 seedlings to total darkness and monitoring the decline in T7RNAP activity levels over time. ST25 was found to be very stable in vivo. Indeed after 16 d in total darkness, SSU-T7 seedlings still retained approximately 50% of the T7RNAP activity present on day zero (Fig. 3B). SSU-T7 plants show increased accumulation of transcripts from genes containing NEP promoters In order to investigate the potential transcriptional consequences of T7RNAP accumulation within chloroplasts, transcript levels for several plastid genes were examined by RNA gel-blot analysis of total leaf RNA extracted from an SSU-T7 plant expressing a high level of T7 RNAP activity (Fig. 4). Two groups of plastid genes were 2346 Magee and Kavanagh Fig. 4. RNA gel-blot analysis of chloroplast mRNA levels in the leaves of wild-type and an SSU-T7 plant expressing a high level of T7 RNAP activity. Equal quantities of total leaf RNA from wild-type (wt) and the SSU-T7 plant (T7) were analysed by denaturing gel electrophoresis. The ethidium bromide-stained gel images are shown for each pair of samples (bottom panels). RNA blots were hybridized with the various named plastid gene probes and the hybridizing bands detected by autoradiography (upper panels). (A) Plastid genes showing increased mRNA levels in SSU-T7 plants. Size markers are given in kb. (B) Plastid genes showing approximately equivalent mRNA levels in the leaves of wild-type and SSU-T7 plants. identi®ed based on a comparison of transcript levels in fully expanded leaves of wild-type and SSU-T7 plants. Group I included plastid genes whose transcripts showed signi®cantly higher levels of accumulation in SSU-T7 plants relative to wild-type plants (Fig. 4A). Genes belonging to this group include rpoC1 which codes for the b¢ subunit of PEP (Shinozaki et al., 1986), rpl33, rps18 and rps12 which encode plastid ribosomal proteins (Shinozaki et al., 1986), and clpP which encodes the proteolytic subunit of the ClpP ATP-dependent protease (Gray et al., 1990; Maurizi et al., 1990). Group II included plastid genes for which similar transcript levels accumulated in both wild-type and SSU-T7 plants (Fig. 4B). This group included psbD which encodes the D2 subunit of photosystem II (Shinozaki et al., 1986), 16S rDNA encoding the plastid 16S rRNA (Shinozaki et al., 1986), and ndhA which codes for a subunit of the plastid NADH dehydrogenase complex (Matsubayashi et al., 1987). The complex polycistronic mRNA patterns typical of most plastid genes was essentially the same in wild-type and SSU-T7 plants despite the considerable increase in transcript levels for group I genes in SSU-T7 plants. One exception to this observation relates to the third smallest transcript (approximately 1.6 kb in size) detected in RNA samples hybridized with the 3¢-rps12 probe (Fig. 4A). The SSU-T7 sample showed a disproportionate increase in the level of this transcript relative to other transcripts. This may have been caused by unequal levels of T7RNAPmediated transcription of the rps12 `split gene' or by increased stability of this transcript in the presence of T7RNAP. Discussion The elimination of PEP activity in DrpoB plants revealed that NEP is responsible for the transcription of genetic Plastid transcripts in tobacco 2347 system genes and some other genes (e.g. clpP), but not the photosystem genes (Allison et al., 1996; Hajdukiewicz et al., 1997). In DrpoB plants, mRNAs from PEPtranscribed genes were absent whereas mRNAs from NEP-transcribed genes showed increased accumulation relative to the levels found in wild-type plants. Promoter mapping studies revealed that genes which contained both PEP and NEP promoters were transcribed from PEP promoters in wild-type plants, but from NEP promoters in DrpoB plants. For this reason it was proposed that the absence of PEP may have initiated a feedback mechanism resulting in increased NEP activity and, consequently, an increase in the abundance of transcripts from genes containing NEP promoters (Hajdukiewicz et al., 1997). In the present study, transgenic plants were produced that accumulate high levels of T7RNAP activity in chloroplasts. These plants show a T7RNAP-dependent increase in the steady-state mRNA levels for genes that are transcribed by NEP. The plastid genes surveyed were representative of the three classes of plastid genes, i.e. genes transcribed from PEP promoters (psbD and ndhA), from both PEP and NEP promoters (16S rDNA, rpl33, rps18, rps12, and clpP) and from NEP promoters alone (rpoC1). Of these increased transcript abundance was found for rpoC1, rpl33, rps18, rps12, and clpP (designated group I genes) in SSU-T7 plants whereas transcript levels for psbD, 16S rDNA and ndhA (designated group II genes) were largely unchanged. Of the group II genes, psbD and ndhA are transcribed exclusively by PEP while the 16S rDNA is transcribed by both PEP and NEP. This suggests that the observed increase in transcript accumulation in the plastids of SSU-T7 plants is speci®c for genes that contain NEP promoters. However, although the tobacco rrn operon contains both a NEP and a PEP promoter (Vera and Sugiura, 1995) there was no apparent increase in 16S rRNA levels in SSU-T7 plants. Indeed DrpoB plants were reported to accumulate reduced levels of 16S rRNA relative to wild-type plants despite the ®nding that two other NEP-transcribed genes analysed (rpl16 and atpI) showed increased transcript levels (Allison et al., 1996). It was proposed that the reduction in 16S rRNA levels in DrpoB plants, despite the presence of a NEP promoter, was caused by the inability of NEP to replace the very high levels of 16S rRNA transcribed predominantly by PEP in the chloroplasts of wild-type plants. In the case of SSU-T7 plants, any increase in 16S rRNA transcript levels may have been too small to be detectable relative to the very high background levels of this transcript. Several hypotheses may be advanced to explain the increased abundance of particular plastid mRNAs in SSUT7 plants. One possibility is that the increases are due to T7RNAP-mediated transcription from NEP promoters. This hypothesis is supported by the following observations (a) T7RNAP shares substantial homology with NEP (Chang et al., 1999), (b) T7RNAP activity is inhibited by anti-NEP antibodies (Bligny et al., 2000) and (c) NEP can initiate transcription from a T7 promoter at least in vitro (Bligny et al., 2000). It therefore seems reasonable to speculate that T7RNAP might also be capable of directing transcription from NEP promoters despite the high sequence speci®city shown by T7RNAP for its cognate phage promoters and the fact that the speci®c amino acid residues which confer promoter recognition in T7RNAP (Rong et al., 1998) are not conserved in the maize NEP enzyme (Chang et al., 1999). It may also be possible that recognition of NEP promoters by T7RNAP is facilitated by transcription factors present in chloroplasts. Alternatively, the presence of T7RNAP activity in chloroplasts may have triggered a feedback mechanism resulting in increased NEP activity as proposed for DrpoB plants (Allison et al., 1996) and recently supported by the observation of a more than 2-fold increase in transcripts of one of the tobacco NEP genes (RpoT3) in DrpoA plants (Hedtke et al., 2002). Increased levels of NEP activity in plastids might, in consequence, result in increased transcription of genes containing NEP promoters. Finally, the possibility cannot be ruled out that the observed increases in transcript abundance might also be the result of increased mRNA stability in SSU-T7 plants, although it is dif®cult to account for a mechanism that would confer increased stability on a speci®c subset of plastid transcripts. SSU-T7 plants containing high levels of T7RNAP activity showed no visible aberrant phenotype, despite the increased levels of some plastid transcripts. This may be due to the fact that T7RNAP expression was restricted to green tissues in SSU-T7 plants. Furthermore, because of the importance of post-transcriptional processes in determining plastid gene expression levels, increased transcript accumulation does not necessarily result in altered levels of the encoded proteins. Acknowledgements We thank Paul Fisher for the gift of rabbit anti-T7 RNA polymerase polyclonal antibodies. We thank John Gray for valuable comments relating to this work. This work was supported by Enterprise Ireland Grant SC/2001/426. References Allison L. 2000. The role of sigma factors in chloroplast transcription. Biochimie 82, 537±548. Allison L, Simon L, Maliga P. 1996. Deletion of rpoB reveals a second distinct transcription system in plastids of higher plants. EMBO Journal 15, 2802±2809. Arnon D. 1949. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiology 41, 1±14. Bartlett S, Grossman A, Chua N-H. 1982. In vitro synthesis and uptake of cytoplasmically-synthesized chloroplast proteins. In: Hallick RB, Chua N-H, eds. Methods in chloroplast molecular biology. New York: Elsevier Press, 1081±1091. 2348 Magee and Kavanagh Baulcombe D, Saunders G, Bevan M, Mayo M, Harrison B. 1986. Expression of a biologically active viral satellite RNA from the nuclear genome of transformed plants. Nature 321, 446±449. Bevan M. 1984. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Research 12, 8711±8721. Bligny M, Courtois F, Thaminy S, Chang C-C, Lagrange T, Baruah-Wolff J, Stern D, Lerbs-Mache S. 2000. Regulation of plastid rDNA transcription by interaction of CDF2 with two different RNA polymerases. EMBO Journal 19, 1851±1860. Bradford M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein±dye binding. Analytical Biochemisty 72, 248±254. Chang C-C, Sheen J, Blingy M, Niwa Y, Lerbs-Mache S, Stern D. 1999. Functional analysis of two maize cDNAs encoding T7like RNA polymerases. The Plant Cell. 11, 911±926. Comai L, Larson-Kelly N, Kiser J, Mau C, Pokalsky A, Shewmaker C, McBride K, Jones A, Stalker D. 1988. Chloroplast transport of a ribulose bisphosphate carboxylase small subunit-5-enolpyruvyl 3-phosphoshikimate synthase chimeric protein requires part of the mature small subunit in addition to the transit peptide. Journal of Biological Chemistry 263, 15104±15109. Dunn J, Krippl B, Bernstein K, Westphal H, Studier F. 1988. Targeting bacteriophage T7 RNA polymerase to the mammalian cell nucleus. Gene 68, 259±266. Fritz C, Herget T, Wolter F, Schell J, Schreier P. 1991. Reduced steady-state levels of rbcS mRNA in plants kept in the dark are due to differential degradation. Proceedings of the National Academy of Sciences, USA 88, 4458±4462. Giuliano G, Hoffman N, Ko K, Scolnik P, Cashmore A. 1988. A light-entrained circadian clock controls transcription of several plant genes. EMBO Journal 7, 3635±3642. Gray J, Hird S, Dyer T. 1990. Nucleotide sequence of a wheat chloroplast gene encoding the proteolytic subunit of an ATPdependent protease. Plant Molecular Biology 15, 947±950. Hajdukiewicz P, Allison L, Maliga P. 1997. The two RNA polymerases encoded by the nuclear and plastid compartments transcribe distinct groups of genes in the tobacco plastids. EMBO Journal 13, 4041±4048. Han C, Patrie W, Polacco M, Coe E. 1993. Aberrations in plastid transcripts and de®ciency of plastid DNA in striped and albino mutants in maize. Planta 191, 552±563. Hedtke B, BoÈrner T, Weihe A. 1997. Mitochondrial and chloroplast phage-type RNA polymerases in Arabidopsis. Science 277, 809±811. Hedtke B, Borner T, Weihe A. 2000. One RNA polymerase serving two genomes. EMBO Reports 1, 435±440. Hedtke B, Legen J, Weihe A, Herrmann R, Borner T. 2002. Six active phage-type RNA polymerase genes in Nicotiana tabacum. The Plant Journal 30, 625±637. Hess W, Prombona A, Fieder B, Subramanian A, BoÈrner T. 1993. Chloroplast rps15 and the rpoB/C1/C2 gene cluster are strongly transcribed in ribosome-de®cient plastids: evidence for a functioning non-chloroplast-encoded RNA polymerase. EMBO Journal 12, 563±571. Horsch R, Fry J, Hoffman N, Eichholtz D, Rogers S, Fraley R. 1985. A simple and general method for transferring genes into plants. Science 227, 1229±1231. HuÈbschmann T, BoÈrner T. 1998. Characterization of transcript initiation sites in ribosome-de®cient barley plastids. Plant Molecular Biology 36, 493±496. Ikeda T, Gray M. 1999. Identi®cation and characterization of T3/ T7 bacteriophage-like RNA polymerase sequences in wheat. Plant Molecular Biology 40, 567±578. Isono K, Shimizu M, Yoshimoto K, Niwa Y, Satoh K, Yokota A, Kobayashi H. 1997. Leaf-speci®cally expressed genes for polypeptides destined for chloroplasts with domains of sigma70 factors of bacterial RNA polymerases in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 94, 14948±14953. Jefferson R, Kavanagh T, Bevan M. 1987. GUS fusions: betaglucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal 6, 3901±3907. Kanamara K, Fujiwara M, Seki M, Katagiri T, Nakamura M, Mochizuki N, Nagatani A, Shinozaki K, Tanaka K, Takahashi H. 1999. Plastidic RNA polymerase sigma factors in Arabidopsis. Plant Cell Physiology 40, 832±842. Kapoor S, Sugiura M. 1999. Identi®cation of two essential sequence elements in the non-consensus type II PatpB-290 plastid promoter by using plastid transcription extracts from cultured tobacco BY-2 cells. The Plant Cell 11, 1799±1810. Kristel E, Inge J, Broekaert W. 1996. High-throughput RNA extraction from plant samples based on homogenization by reciprocal shaking in the presence of a mixture of sand and glass beads. Plant Molecular Biology Reporter 14, 273±279. Laemmli UK. 1970. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227, 680±685. Lerbs-Mache S. 2000. Regulation of rDNA transcription in plastids of higher plants. Biochimie 82, 525±535. Liere K, Maliga P. 1999. In vitro characterization of the tobacco rpoB promoter reveals a core sequence motif conserved between phage-type plastid and plant mitochondrial promoters. EMBO Journal 18, 249±257. Maniatis T, Fritsch E, Sambrook J. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbour Laboratory Press. Matsubayashi T, Wakasugi T, Shinozaki K, et al. 1987. Six chloroplast genes (ndhA-F) homologous to human mitochondrial genes encoding components of the respiratory chain NADH dehydrogenase are actively expressed: determination of the splice sites in ndhA and ndhB pre-mRNAs. Molecular and General Genetics 210, 385±393. Maurizi M, Clark W, Kim S, Gottesman S. 1990. Clp P represents a unique family of serine proteases. Journal of Biological Chemistry 265, 12546±12552. Mazur B, Chui C. 1985. Sequence of a genomic DNA clone for small subunit of ribulose bis-phosphate carboxylase-oxygenase from tobacco. Nucleic Acids Research 13, 2373±2386. McBride K, Schaaf D, Daley M, Stalker D. 1994. Controlled expression of plastid transgenes in plants based on a nuclear DNA-encoded and plastid-targeted T7 RNA polymerase. Proceedings of the National Academy of Sciences, USA 91, 7301±7305. Odell J, Nagy F, Chua N-H. 1985. Identi®cation of DNA sequences required for activity of the CaMV 35S promoter. Nature 313, 810±812. Oikawa K, Fujiwara M, Nakazato E, Tanaka K, Takahashi H. 2000. Characterization of two plastid s factors, SigA1 and SigA2, that mainly function in matured chloroplasts in Nicotiana tabacum. Gene 261, 221±228. Rong M, He B, McAllister W, Durbin R. 1998. Promoter speci®city determinants of T7 RNA polymerase. Proceedings of the National Academy of Sciences, USA 95, 515±519. Sambrook J, Fritsch E, Maniatis T. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbour Laboratory Press. Silhavy D, Maliga P. 1998. Mapping of promoters for the nucleusencoded plastid RNA polymerase (NEP) in the iojap maize mutant. Current Genetics 33, 340±344. Shinozaki K, Ohme M, Tanaka M, et al. 1986. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO Journal. 5, 2043±2049. Sriraman P, Silhavy D, Maliga P. 1998. The phage-type PclpP-53 Plastid transcripts in tobacco 2349 plastid promoter comprises sequences downstream of the transcription initiation site. Nucleic Acid Research 26, 4874± 4879. Sushmita D, Lahiri S, Allison L. 2000. Complementary expression of two plastid-localized s-like factors in maize. Plant Physiology 123, 883±894. Tanaka K, Tozawa Y, Mochizuki N, Shinozaki K, Nagatani A, Wakasa K, Takahashi H. 1997. Characterization of three cDNA species encoding plastid RNA polymerase sigma factors in Arabidopsis thaliana: evidence for the sigma factor heterogeneity in higher plant plastids. FEBS Letters 413, 309±313. Vera A, Sugiura M. 1995. Chloroplast rRNA transcription from structurally different tandem promoters: an additional novel-type promoter. Current Genetics 27, 280±284.