* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Mutator Transposon in Maize and MULEs in the Plant Genome
Koinophilia wikipedia , lookup
Copy-number variation wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Population genetics wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Gene desert wikipedia , lookup
Whole genome sequencing wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Gene expression programming wikipedia , lookup
Genomic imprinting wikipedia , lookup
Genetically modified crops wikipedia , lookup
Public health genomics wikipedia , lookup
Genetic engineering wikipedia , lookup
Gene expression profiling wikipedia , lookup
Metagenomics wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
Oncogenomics wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Genomic library wikipedia , lookup
Short interspersed nuclear elements (SINEs) wikipedia , lookup
Point mutation wikipedia , lookup
Human Genome Project wikipedia , lookup
Pathogenomics wikipedia , lookup
Human genome wikipedia , lookup
Genome (book) wikipedia , lookup
Non-coding DNA wikipedia , lookup
Designer baby wikipedia , lookup
History of genetic engineering wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Minimal genome wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Microevolution wikipedia , lookup
Genome editing wikipedia , lookup
Transposable element wikipedia , lookup
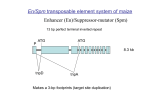
遗 传 学 报 Acta Genetica Sinica, June 2006, ISSN 0379-4172 33 (6):477–487 Mutator Transposon in Maize and MULEs in the Plant Genome DIAO Xian-Min1,①, Damon Lisch2 1. National Millet Improvement Center of China, Institute of Millet Crops, Hebei Academy of Agricultural and Forestry Sciences, Shijiazhuang 050031, China; 2. University of California at Berkeley, Department of Plant and Microbial Biology, 111 Koshland Hall, CA 94720, USA Abstract: Mutator (Mu) is by far the most mutagenic plant transposon. The high frequency of transposition and the tendency to insert into low copy sequences for such transposon have made it the primary means by which genes are mutagenized in maize (Zea mays L.). Mus like elements (MULEs) are widespread among angiosperms and multiple-diverged functional variants can be present in a single genome. MULEs often capture genetic sequences. These Pack-MuLEs can mobilize thousands of gene fragments, which may have had a significant impact on host genome evolution. There is also evidence that MULEs can move between reproductively isolated species. Here we present an overview of the discovery, features and utility of Mu transposon. Classification of Mu elements and future directions of related research are also discussed. Understanding Mu will help us elucidate the dynamic genome. Key words: Mutator; transposon; genome evolution; MULE; Pack-MuLE Transposons or “controlling elements” were discovered by Barbara McClintock in maize (Zea mays L.) in the early 1950s, but the theory of transposition was not widely accepted until 1970s. Transposable elements of one type or another have been found in all organisms, including all plants that have been investigated. Transposons make up over 50% DNA of the genome in many species with large genomes. Transposon can rearrange genomes and alter individual gene structure and expression as a consequence of transposition, insertion, excision and chromosome breakage. Many transposon system have been studied in China, such as Ac/Ds, Spm/dSpm, Tourist, Stowaway in maize, Mariner in soybean (Glycine max L.) and Tam in Snapdragon[1,2]. However, little is known about Mutator (simplified as Mu), which is the hot point of transposon research in America. Mutator transposon was discovered by Robertson in 1978 from a maize line that yielded diverse mutations at a frequency much higher than the spontaneous mutation frequency [3, 4]. It was found that these mutations were caused by the insertion of Mu transposons. Mu elements have be- come the major tool of gene discovery in maize functional genome research and other related fields. Here we review the discovery, properties, genetic application of Mu transposon and its role in plant gene and genome evolution. 1 The Discovery of Mu Transposon A maize line, known as Mutator, was sent to D. S. Robertson of Iowa State University from the University of Wisconsin for detailed analysis of its frequent mutations. Genetic experiment by Robertson demonstrated that not only the heritable mutation frequency of this line was very high, but a great deal of somatic variation was also observed. Further research showed that these mutations were not caused by the previously discovered transposons such as Ac/Ds and Spm/dSpm. In Ac/Ds and Spm/dSpm lines, only a few autonomous elements are present and they segregate as a Mendelian element. Transpositions of these elements, when they occurred, could be detected as changes in the segregation ratios of activity. Received: 2005-09-21; Accepted: 2005-10-08 This work was supported by the National Natural Science Foundation of China (No.30370766). ① Corresponding author. E-mail: [email protected]; Tel: +86-311-8767 0697 478 In contrast, 90% of the progeny of an outcross of a Mutator line carried active transposons and were consistent with a very high duplication rate. Those progenies that did lose activity appeared to do so due to epigenetic silencing, rather than the segregation of an autonomous, or controlling element. This was confirmed in many later experiments [5,6]. The complexity and epigenetic regulation features of this putative transposon system delayed the discovery of the means by which the system was regulated. In the 1980s, using this Mutator line as mutagen for gene tagging became popular. A new kind of transposon inserted into an allele of alcohol dehydrogenase1 and was cloned by Bennetzen et al [7]. Now we know that this transposon is a nonautonomous member of the Mu transposon family, which was named Mu1. Characterization of Mu1 and using it as a probe not only identified a new kind of transposon but also opened a new era of efficient maize functional genome research. This new transposon was named Mutator because it came from Mutator maize line. Schnable et al. [8] identified a Cy/rcy transposon system from an independent maize line with similar transposion and genetic properties of Mu system, which was later demonstrated to be Mu7 of the Mu family. In the early 1990s, a minimal version of the Mutator line was used to isolate the regulatory transposon for the Mutator system. This regulator, which was isolated independently in three different laboratories, was designated as MuDR, in honor of Don Robertson. The isolation of the autonomous two-element, MuDR [9, 10], the creation of a minimal Mutator line [11] and an engineered rescue-Mu element [12] have made it possible for a systematically elucidation of this super transposon family. 2 The Components and Gene Structure of MuDR and Mu Transposon Family All maize Mu-transposable elements are regulated by MuDR, which is the autonomous element; MuDR carries two genes, mudrA and mudrB (Fig. 1). The mudrA transcript encodes a 120 kDa protein, MURA, which is the transposase of the family. MURA contains a domain with high similarity to several bacterial 遗传学报 Acta Genetica Sinica Vol.33 No.6 2006 transposase, which was used as evidence for its very early origin [13]. The mudrB major transcript encodes a 23 kDa protein (MURB) that is not similar to any sequences in public database outside maize and its close relatives. Although the precise function of MURB remains enigmatic, both deletion derivatives and transgenic experiments suggest that MURB is required for Mu insertions, especially germinally transmitted insertions [14]. Only two maize lines with active MuDR elements have been identified so far, but all maize lines carry MuDR elements derivatives, or homologous MuDR sequences (hMuDRs), whose coding sequences are 80%–99% identical to those of MuDR. Surprisingly, these hMuDRs can be expressed at the transcription level though they are not associated with Mutator activity and they might play an as-yet-unknown function in Mutator regulation [5]. Unlike Ac/Ds and Spm/dSpm transpson systems, which mostly comprise of the autonomous element and its deletion derivatives, the Mu transposon system contains complex family elements, including autonomous MuDR, homologous MuDR elements and many variants with similar TIRs. All Mu transposon elements share similar -170-220 bp TIRs; the length of direct repeat sequence flanking the inserted elements is 9 bp. Many Mu elements contain additional direct or inverted repeat sequences. Although deletion derivatives are the most common source of Ds element from Ac and of dSpm elements from Spm, most Mu elements internal sequences are unrelated to the tranposase-coding sequence of MuDR, or to each other (Fig. 1). In most cases, the internal sequences of Mu elements are part of a host gene and it seems that Mu TIRs can capture host functional sequence by so far unknown mechanism. These kinds of elements are called Pack-MuLEs [15]. In some species, such as rice (Oryza sativa L.), there can be thousands of independently derived Pack-MuLEs. Jittery is a newly isolated and characterized member of Mu element, whose transposase-coding sequence is only a 3.9 kb gene with high identity to mudrA but not to mudrB. The length of direct host sequence duplication flanking the Jittery insertions is also 9 bp, but its 177 bp TIRs are unrelated to that of DIAO Xian-Min et al.: Mutator Transposon in Maize and MULEs in the Plant Genome 479 Fig. 1 The Mutator transposon family in maize and the engineered RescueMu All classes of Mu elements share similar terminal inverted repeats (TIRs; Black ends), but each class has a unique internal sequence that shares similarity to various host functional protein coding sequences, as indicated in the figure. Mu1 is similar to MRS-A (Mu-related sequence A). Mu3 is similar to an Arabidopsis protein and Mago nashi protein. Mu4 includes a sequence 95% identical to a maize expressed sequence, accession No. BG466445. Mu7 is similar to Arabidopsis protein accession No. NP-192120. Mu8 contains a sequence with similarity to pgp1 protein from Arabidopsis. RescueMu is an engineered element based on Mu1 used as a gene-tagging tool, into which unique tag sequence, pBluescript and selectable maker were inserted. For MuDR, the position of mudrA and mudrB are indicated. The figure is a modified version from Lisch (2002) [17]. MuDR. Despite limited investigation has been done on the transposition activity of Jittery, the results so far obtained demonstrated that it is an autonomous transposable element. Jittery, like MuDR causes a high frequency of late somatic reversion, but like deletion derivatives that lack mudrB, it is not associated with new insertions [16]. In addition to the members of Mu elements found in maize, many Mu-like elements (MULEs) have been identified in both dicots and monocots. Mu transposon family is characterized by the presence of many diversified and potentially functional variants, and it’s very high levels of activity in maize, which complicated analysis of its origin and evolution. 3 Transposition Activity of Mu Data related to Mu transposition activity are largely a product of investigations on maize, although MULEs in Arabidopsis had been confirmed to be active in DDM1 mutant background [18]. Two main types of transpositions occur with maize Mu elements. One is somatic excision, or excision and insertion late during development in somatic cells and tissues, the other one is insertions occurring in germinal cells, which is the source of heritable new mutations in Mutator lines. Mu excisions have been investigated extensively with anthocyanin reporter alleles, such as a1-mum2, because pigmented spots can easily be visualized and quantified. Most somatic excisions occur in the late development stage, within the last two or three cell divisions of a given lineage, though early excisions can occasionally occur. The most visible result of somatic excision is revertant sectors, resulting from the excision of the inserted transposon and restoration of the a1 pigmentation function. Two kinds of somatic excision have been documented for Mu elements, the cut-only and the cut-and-paste. The excised transposons of the cut-only form were failed in inserting into other sites of the host genome. Cut-and-paste transposition is the typical mode of transposition of DNA transposons and is often associated with reinsertion elsewhere in the genome. But excision of Ac/Ds and Spm/dSpm elements typically result in minor changes to the host sequence duplication created on transposon insertion. In contrast, Mu element excision create deletions and additions of the host gene[19, 20]. In contrast to high frequency of somatic revertant 480 mutations, germinally transmitted revertants are extremely rare in Mutator lines, which suggest that excisions are prevented by some kind of mechanism in cells that give rise to gametes[21]. In minimal Mutator lines with just one copy of Mu1 initially inserted into the a1-mum2 allele and one copy of MuDR, from 10%–20% of progeny contain more than one copy of Mu1 or MuDR[11] and many experiments conformed that the existing Mu insertion segregate in a Mendelian fasion. These experiments reveal that new germinal insertions must happen without the loss of the existing Mu insertion; in another word, some kind of duplicative or replicative MuDR transposition happens during the process of development[5, 18]. Though germinal duplicative insertion can occur throughout the development, which mostly begins late in sporophyte cell divisions, where transposition events create small clusters of gametes carrying the same mutations. Germinal transposition continue through meiosis to the last mitotic divisions of the gametophytes, and the after meiosis insertion generates sperm with different mutations [3,22]. The frequency of germinally transmitted insertions depends on the Mutator line used, but there are clear position effects on both cis and trans activity of individual MuDR elements [17]. Many Mu-induced mutations are suppressible. The mutant phenotype in these cases arises only in the presence of active MuDR elements, probably due to steric effects introduced by the presence of the transposase bound to the transposon TIRs. Consistent with this model, many suppressible Mu insertions are in promoter regions. However, Mu insertion into introns can also be suppressible [23], and it is likely that Mu suppression will turn out to be more complex than a simple model of steric hindrance would suggest. Many investigations have demonstrated that Mu transposons generally insert into single copy or low-copy-number regions of the genome [24], which makes it a powerful tool for maize functional gene tagging. By analyzing the sequences that flanking the 88 RescueMu insertions, Dietrich and co-authors demonstrated that 69% of these sequences were genes and only 4% were repetitive retrotransposons[25]. A much recent experiment also with RescueMu shows that the rate of 遗传学报 Acta Genetica Sinica Vol.33 No.6 2006 single copy insertion is about 66%, which is consistent with the previous result [26]. Both Ac/Ds and Spm/dSpm tend to transpose to genetically linked sites. In contrast, Mutator transposes to unlinked sites, an advantage for whole genome mutagenesis applications. However, Hardeman and Chandler[27] found that certain classes of Mu elements predominantly targeted certain genes, which implies that some Mu elements may have insertion affinities. The 5′UTR region or the promoter region of gl8 gene showed a strong preference for Mu element insertion, 62 of 75 insertions of gl8 gene targeted in this region, suggesting that 5′UTR targeting might be a feature of Mu insertion. But introns and other regions of genes are also often targeted by Mu elements[25]. Analysis of 339 target site duplications (TSDs) created by Mu insertions also showed some degree of sequence preference, the weak consensus for Mu insertion was CTCB(G/C)(A/C)(G/A)(A/G)C. Furthermore, sequences immediately linked to TSDs also showed conservation; the consensus sequence of 5′ of the TSD is CCT and that of the 3′ of the TSD is AGG. Mu-targeted sequences were found to be GC rich relative to the rest of the maize genome [25]. However, many Mu insertion sites do not have the consensus sequence. Four types of assays are usually used to demonstrate Mutator system activity, including examination of somatic instability of reporter alleles (such as sectored leaves, kernels or anthers), special enzymes to detect methylation of diagnostic restriction sites in the TIRs (HinfⅠfor Mu1 and SacⅠfor MuDR), the detection of new insertions in progeny plants, and an elevated forward mutation frequency resulting from new germinal insertions[5]. Which method should be used depends on needs of the user. Early observations of Mutator suggested that the loss of activity was due to epigenetic silencing, rather than simple segregation of a regulatory transposon. Two models for silencing had been proposed: ectopic paring between homologous TIRs and posttranscriptional RNA-based silencing [5,28]. Until recently, the detailed initiation and process of Mu family silencing still remains enigmatic. A dominant locus that can initiate Mutator silencing, Mu killer (Muk), was cloned. This locus was demonstrated to be an inverted duplication of a partially deleted autonomous MuDR element. Muk produces a mudrA hairpin DIAO Xian-Min et al.: Mutator Transposon in Maize and MULEs in the Plant Genome transcript that is processed into small RNAs that targets mudrA for silencing. This, in turn, results in transcriptional silencing of mudrA, and subsequently mudrB. Muk provides the first example of a natural occurrence of derivative that is able to initiate heritable silencing of an active transposon family [29, 30]. This discovery not only clarified our understanding of Mu epigenetic silencing, but also gave us new tools for investigation of both transcriptional and posttranscriptional regulation of gene expression. It is suggested that the initial silencing of Mu activity is followed by detectable TIRs methylation[5]. Indeed, 5′-methylation of cytosines within TIRs is a diagnostic feature of Mu transposons silencing state. In typical Mutator lines or complex lines, both MuDR and nonautonomous element, such as Mu1, can be methylated gradually in the progeny of a linage or at different development stage of an individual plant. The methylation of MuDR is accompanied by transcriptional silencing and loss of activity [5,31]. In minimal lines with single copy of MuDR, both MuDR and the nonautonomous elements are unmethylated. Methylation of nonautonomous elements occurs if MuDR element is lost due to genetic segregation. In such cases that MuDR is restored genetically, the methylation of nonautonomous elements is lost, suggesting that it represents a default state that occurs in the absence of the transposase[17, 32]. This default methylation is dependent on at least two mutations that were discovered due to their effects on paramutation. In mop1 (mediator of paramutation) mutants, paramutatable alleles of several color genes are epigenetically activated and nonautonomous Mu elements 481 are hypomethylated. MuDR elements that had been silenced by Mu killer are also hypomethylated in a mop1 mutant background. If this element is maintained in a mutant background for multiple generations, one of the two genes encoded by MuDR, the transposase mudrA becomes reactivated. Further, the second gene, mudrB, remains methylated and silenced, suggesting that, although the two genes had both been silenced by Muk, maintenance of that silenced state is mediated by different factors[33]. Silenced Mu elements in typical lines can also be reactivated by gamma and UV radiation of seeds and pollen, and radiation treatments are more efficient on lines within one or two generations of silencing than those lines that had kept many generations of silence state[5]. Singer et al. [18] found that Mutator-like elements (MULEs) in Arabidopsis genome become demethylated and active in the chromatin-remodeling mutant ddm1 (Decrease in DNA Methylation), which leads to loss of heterochromatic DNA methylation. 4 Genetic Application of Mu Transposon Transposon tagging is one of the main methods used in functional genome research. Ac/Ds tagging system has not only been successfully used in maize but also in rice and Arabidopsis [34]. The advantages of Mu tagging are that it moves to any chromosome, it causes a very high mutation rate, insertions tend to be into or near genes, and most loci appear to be potential targets. Using this transposon system, many maize genes have been cloned and characterized, some of which are listed in Table 1. Table 1 List of some Mu tagging cloned maize functional genes No. Gene or allele Possible function Database code Reference 1 Su1 Kernel development related AY290402 [35] 2 Rough sheath1 Leaf sheath development related L44133 [36] 3 APETALA2 Spikelet development related AF048900 [37] 4 Ligueless3 Architecture of ligule AF457125 [38] 5 Knotted1 Leaf development AY312169 [39] 6 Viviparous1 Induction of embryo development AJ001635 [40] 7 Adh1 Oxygen starvation response X04049 [7] 482 Targeted mutagenesis was the initial application in typical Mutator lines, because it is relatively easy for scientists to recover mutations in well-studied genes that confer visible phenotypes [5]. For loci with a recessive loss-of-function allele, wild-type plant with active Mu were crossed to the homozygous recessive tester stock, mutant individuals in the F1 progeny would be expected to carry a Mu-tagged allele from the Mu-active parent. For loci with dominant gain-of-function alleles, Mu-active lines homozygous for the dominant allele need to be established first, and then crossed to a normal test line. Normal individuals of the F1 most likely contained a Mu-inserted disruption of the dominant allele. Because Mu causes a high mutation rate, large populations of Mu active lines have been screened to find new phenotypes of interest. Three big project of Mu-tagging maize functional genome research had been carried out in the United States. The Trait Utility System for Corn (TUSC) was implemented by Pioneer Hi-Bred, Inc., which was based on DNA samples of ~45 000 Mu-active individual plants and the available of maize EST sequences. Using a primer running out from the Mu TIR sequence and a primer designed on the basis of an EST of interest, population screening can be used to find individuals with a Mu insertion into or near the gene of interest. Progeny analysis can then be used to determine which plants transmitted a heritable mutant allele. Maize-Targeted Mutagenesis (MTM) is a US National Science Foundation funded project for public maize research service. With the same strategy to TUSC, MTM uses PCR to screen from a population of ~46 000 Mu active plants and provide users with seed for phenotypic characterization. RescueMu was another project carried out by joint co-operation of Stanford University, UC Berkeley and other institutes, which involves screening progeny of RescueMu transformed maize plants. This approach has the virtue of combining genomic sequencing and functional genomics into a single step, in which RescueMu insertions provide not only a mutation but also a cloned allele for sequencing. Since Mu transposon had been effectively used 遗传学报 Acta Genetica Sinica Vol.33 No.6 2006 in maize functional genome research, it would be advantageous if we use this system in other plant species for gene tagging as well. The most direct way to do this is to create a heterologous Mu transposon tagging in other species. Unfortunately, although mudrA and mudrB have been successfully transformed into rice, and the transformed genes can be inherited for a few generations, transcription of mudrA and mudrB was not detected (unpublished data). So we need more knowledge about Mu epigenetic regulation and transferring gene silencing. Transposition active MULEs have been observed in Arabidopsis DDM1 mutant [18] and a fungal species Fusarium oxysporum [41], suggesting that transpositionally active Mu elements do exist in organisms in addition to maize. Thus, screening for active and mutagenic MULEs may be another option for Mu tagging in species such as rice. 5 Occurrence and Classification of Mu Elements in Other Plant Genomes It has been demonstrated that homologs of mudrA exist in many plant genomes, but homologs of mudrB have only been observed in Z. luxurians, Z. diploperennis and a few other species closely related to maize[42]. Mu-like elements are generically referred to as MULEs[15]. Autonomous MULEs show varying degrees of similarity with mudrA in maize. So far intact element of MULEs, including TIRs and TSDs, had been cloned and characterized from Arabidopsis, Brassic napus, rice, sorghum (Sorghum bicolor L.), sugar cane (Saccharum officinarum L.), wheat (Triticum aestivum L.), barley (Hordeum vulgare L.), foxtail millet (Setaria italica L.) and a few bamboo species (Bambuseae) species[5, 42-44]. Lisch et al.[42] cloned and characterized mudrA sequences from 28 plant species. Cluster analysis based on the unclear tide identity of the cloned fragments showed a discrepancy in the relationship between phylogeny of the sample species and the evolution phylogeny of those MULEs. Eisen et al. [13] found that the sequence of transposase mudrA was similar to transposase of some insertion sequence (IS) in a group of bacteria DIAO Xian-Min et al.: Mutator Transposon in Maize and MULEs in the Plant Genome species such as Mycobacterium bovis. These suggest that the Mu transposon family might have had a very early origin. The genomes of fungus do have MULEs and even a transpositional active one was characterized [41] . To date no MULEs have been reported in animals. Not only do we know that MULEs are ubiquitous in plant genomes so far tested, but analysis of public EST data demonstrate that MULEs in many plants, including rice, wheat, sugar cane and barley are transcripted. Thus, transcriptional active MULEs maybe a common property of plant genomes[42]. Most MULEs contain mudrA genes that are well diverged from that in maize, but many of those MULEs have good open-reading frames with evidence for selection at the amino acid level, consistent with continued functionality. Conservation of TIR sequences with each other and targeted side duplication (TSD) is also constant with continued transposition activity. Many duplicated copies of one kind MULEs were found in the sequenced rice genome (unpublished observation), and we found several MULEs of Setaria viridis were highly polymorphic among different genotypes of the same species by Southern blot (unpublished data), suggesting activity in this species. Database search using the protein sequence of MURA reveals that proteins with high similarity to MURA exist in Arabodopsis, potato (Salanum tuberosum L.), tomato (Lycopersicon esculentum M.) and many other plant species [42]. Yoshida [45] found a rice MULE expressed in callus subcultured with praline and even found a transcriptionally active MULE in rice somaclonal lines. Three instances of transpositionally active MULEs have been reported, including Jittery in maize, AtMu1 in Arabidopsis DDM mutant and Hop1 in the fungus Fusarium oxysporum. Jittery resembles Mutator in the length of the element’s TIRs, the size of the target site duplication, and the makeup of its transposase, but differs from MuDR in that it encodes a single MURA-like protein. Jittery also differs from Mutator elements in the high frequency with which it excises to produce germinal revertants and in its low copy number in most maize lines and maize relatives examined. However, Jittery cannot be considered as a bona fide transposon in its present host line, because 483 it does not reinsert in the genome [16]. Together, these data suggest that MULEs may be transpositionally active now or in the near past in many plant genomes. Thus, it is quite likely that active Mu systems in other plant species may be as useful as Mutator does in maize. MULEs can be widely diverged from each other even they come from the same genome. Jittery, which is an active MULE in maize, is more similar to MULEs in rice and Arabidopsis than it is to MuDR [16] . Re- markably, two of MULEs in Arabidopsis with high similarity to Jittery appear to be host functional genes rather than transposons; mutations in those genes cause defects in the far-red-light response pathway [46-48] . In this case, it appears that the transposase has been recruited by the host to perform a novel function. The distribution of any given group of MULEs in the grasses is patchy, suggesting the possibility of horizontal gene transfer. Diao and colleagues[49] found a MULE from Setaria with high similarity to a MULE from rice; the nucleotide identity between the two elements is as high as 90% including corresponding intron sequences. Given the 50–60 million years of evolution divergence that separate Setaria and rice[50], the high degree of similarity of non-coding sequences can only be explained by horizontal transfer[49]. This is the first well-documented example of horizontal transfer of any nuclear-encoded genes between higher plants. It is clear that the evolution of Mu super family is markedly different from their hosts. MULEs form a highly complex and broadly diversified family of transposon. All Mu-related elements so far found in plant genomes can be classified into four groups: the first includes MuDR and hMuDRs as stated in the second part of the review. Many of these elements contain point mutations but are transcriptionally expressed and may play a role in the epigenetic regulation of the family [5]. The second group includes elements that have mudrA homologs and long distinct TIRs from MuDR, such as Jittery in maize and Sf4 in Setaria. The third group of Mu super family includes those elements that share similar TIRs with a MULE encoding mudrA but that carry internal sequences that are unrelated to the 484 transposase. Examples of these include some of the nonautonmous elements in maize. Their internal sequences are captured fragments of host genes. These elements are known as Pack-MULEs, and they can be present in large numbers in any given genome [15, 51, 52]. The fourth group includes transposons that carry mudrA-like genes but lack long TIRs. Individual instances of this class of elements may represent coopted mudrA genes, genes such as Far1 in Arabidopsis [46, 47]. 6 Mu Elements in Plant Genome and Gene Evolution The genome of all plant species is in a state of dynamic equilibrium between evolution and stability, but of course stability is always temporary. The diverse activity of transposons has been demonstrated to be an important factor affecting on the evolution of genes and genomes [1,53,54]. Transposon can induce gene silencing or reactivation, gene or genome restructuring including deletion, duplication, reversion and translocation. The accumulation of such changes undoubtedly has had a profound impact on genome evolution. Mu transposons are the most active, most complex and most ubiquitous DNA transposable element in plant genomes, suggesting a special role in gene and genome evolution. Unlike Ac/Ds and Spm/dSpm, whose activity result in relatively minor changes to the host sequence, Mu activity can result in a wide range of changes[12]. It has demonstrated that MuDR elements in different loci can show distinct frequencies of transposition, suggesting that this class of elements is particularly sensitive to their local environment. It is clear that Mu elements can evolve a novel function, such as in the case of the Arabidopsis Far1 gene [46, 47]. The most recent discovery of the impact of Mu elements on plant genome evolution is Pack-MULEs, which contain fragments of genes. With systematic and careful analysis, Jiang et al.[55, 56] fund more than 3 000 Pack-MULEs in the rice genome, and Pack-MULEs also had been seen in maize and Arabidopsis. The large number of Pack-MULEs and their presence in multiple genomes suggest that they may be a major component of most or all flowering plant genomes. 遗传学报 Acta Genetica Sinica Vol.33 No.6 2006 Gene fragments from different rice genes were found together in -23% of Pack-MULEs in rice, suggesting a means by which hybrid genes could be created due to the activity of a transposon. At least 5% of Pack-MULEs were found to be expressed, as evidenced by full-length cDNAs, with an identical DNA sequence match. Hence, by the criterion of expression at the RNA level, many of these Pack-MULEs are already new genes. The ability of Pack-MULEs to capture gene fragments combined with the possibility of horizontal transfer as documented by Diao et al.[49] also suggest a means by which gene fragments could be moved between species. Although we have made some progress in understanding of the MuDR/Mu superfamily, many interesting puzzles remains to be explored for future research. The most intriguing questions include the detail mechanism of Mu transposition and the epigenetic regulation of Mu silencing and reactivation. The solution for such questions will help us establish Mu tagging system in other plants than maize and widening its application in plant genetics. The frequent transposition and interaction with host genomes make the evolution study of transposons difficult, but analysis of this fascinating super family of elements and a clear understanding of their evolution will certainly help us understand the dynamics of gene and genome evolution. Pack-MULE structure is ubiquitous in plant genomes and predicted to be a mechansism for the creation of novel genes[51, 52], but we know nothing about the mechanism of fragments acquisition and so far no instances of newly created genes in this way have been reported. As stated by Shapiro of Chicago University[54] “transposable elements are the key to a 21st century view of evolution”, advances in our understanding of the MULE super family will for certainly help us to illuminate both transposon and genome evolution. Acknowledgement: We thank Hui Zhi of National Millet Improvement Center of China for figure preparation. References: [1] Bennetzn J L. Transposable element contributions to plant gene and genome evolution. Plant Mol Biol, 2000, DIAO Xian-Min et al.: Mutator Transposon in Maize and MULEs in the Plant Genome 42(1) : 251-269. [2] SanMiguel P, Bennetzn J L. Evidence that a recent increase in maize genome size was caused by the massive amplification of intergene retrotransposons. Ann Bot Res, 1998, 82(Supplement A) : 37-44. [3] Robertoson D S. Mutator activity in maize: timing of its activation in ontogeny. Science, 1981, 213(4515) : 1515-1517. [4] Robertoson D S. Genetic studies on the loss of Mu mutator activity in maize. Genetics, 1986, 113(3) : 765-773. [5] Walbot V, Rudenko G N. MuDR/Mu transposons of maize. In: Aig N L, Craigie R, Gellert M, Lambowitz A, eds. Mobile DNAⅡ. American Society for Microbiology, Washington, DC. 2002, 533-564. [6] Martienssen R, Baron A. Coordinate suppression of mutations caused by Robertson’s mutator transposons in maize. Genetics, 1994, 136 (3) : 1157-1170. [7] Bennetzen J L, Swanson J, Taylor W C, Freeling M. DNA insertion in the first intron of maize Adh1 affects message levels: cloning of progenitor and mutant Adh1 alleles. Proc Natl Acad Sci USA, 1984, 81(13) : 4125-4128. [8] Schnable P S, Peterson P A. The Mutator-related Cy transposable element of Zea mays L. behaves as a tion in plants. Nature, 2004, 431(7008) : 569-573. [16] Xu Z N, Yan X H, Maurais S, Fu H H, O’Brien D G, Mottinger J, Dooner H K. Jittery, a Mutator distant relative with a paradoxical mobile behavior: excision without reinsertion. Plant Cell, 2004, 16(5) : 1105-1114. [17] Lisch D. Mutator transposons. Trends Plant Sci, 2002, 7(11) : 498-504. [18] Singer T, Yordan C, Martienssen R A. Robertson’s Mutator transposons in A. thaliana are regulated by the chromatin-remodeling gene Decrease in DNA Methylation (DDM1). Genes Dev, 2001, 15(5) : 591-602. [19] Nordborg M, Walbot V. Estimating allelic diversity generated by excision of different transposon types. Theor Appl Genet, 1995, 90(6): 771-775. [20] Britt A B, Walbot V. Germinal and somatic products of excision of Mu1 from the Bronze-1 gene of Zea mays. Mol Gen Genet, 1991, 227(2) : 267-276. [21] Chandler V L, Hardeman K J. The Mu elements of Zea mays. Adv Genet, 1992, 30 : 77-122. [22] Robertoson D S. The timing of Mu activation in maize. Genetics, 1989, 94(4) : 969-978. [23] Greene B, Walko R, Hake S. Mutator insertions in an intron of the maize Knotted1 gene result in dominant near-Mendelian factor. Genetics, 1988, 120(2) : 587-596. suppressible [9] Chomet P, Lisch D, Hardeman K J, Chandler V L, Freeling M. Identification of a regulatory transposon that controls the Mutator transposable element system in maize. 1275-1285. Genetics, 1991, 129(1) : 261-270. [10] Hershberger R J, Warren C A , Walbot V. Genetics Mutator activity in maize correlates with the presence and expression of the Mu transposable element Mu9. Proc Nail Acad Sci USA, 1991, 88(22) : 10198-10202. [11] Lisch D, Chomet P, Freeling M. Genetic characterization of the Mutator system in maize: behavior and regulation of Mu transposons in a minimal line. Genetics, 1995, 139(4) : 1777-1796. [12] Raizada M N, Nan G L, Walbot V. Somatic and germinal mobility of the RescueMu transposon in transgenic maize. Plant Cell, 2001, 13(7) : 1587-1608. [13] Eisen J A, Benito M I, Walbot V. Sequence similarity of putative transposases links the maize Mutator autonomous element and a group of bacterial insertion sequences. Nucleic Acids Res, 1994, 22(13) : 2634-2636. [14] Lisch D, Girard L, Donlin M, Freeling M. Functional analysis of deletion derivatives of the maize transposon MuDR delineates roles for the MURA and MURB proteins. Genetics, 1999, 151(1) : 331-341. [15] Jiang N, Bao Z R, Zhang X Y, Eddy S R, Wessler S R. Pack-MULE transposable elements mediate gene evolu- 485 mutations. Genetics, 1994, 138(4) : [24] Fernandes J, Dong Q, Schneider B, Morrow D J, Nan G L, Brendel V, Walbot V. Genome-wide mutagenesis of Zea mays L. using RescueMu transposons. Genome Biol, 2004, 5(10) : R82 [25] Dietrich C R, Cui F, Packila M L, Li J, Ashlock D A, Nikolau B J, Schnable P S. Maize Mu transposons are targeted to the 5 untranslated region of the g18 gene and sequences flanking Mu target-site duplications exhibit nonrandom nucleotide composition throughout the genome. Genetics, 2002, 160(2) : 697-716. [26] Fernandes J, Dong Q F, Schneider B, Morrow D J, Nan G L, Brendel V, Walbot V. Genome-wide mutagenesis of Zea mays L. using RescueMu transposons. Genome Biol, 2004, 5(10) : R82. 1-R82.20. [27] Hardeman K J, Chandler V L. Two maize genes are each targeted predominantly by distinct classes of Mu elements. Genetics, 1993, 135(4) : 1141-1150. [28] Bennetzen J L, Springer P S, Cresse A D, Hendrichkx H. Specificity and regulation of the Mutator transposable element system in maize. Crit Rev Plant Sci, 1993, 12(1) : 57-95. [29] Slotkin R K, Freeling M, Lisch D. Mu killer causes the heritable inactivation of the Mutator family of transposable elements in Zea mays. Genetics, 2003, 165(2) : 781-797. 486 [30] Slotkin R K, Freeling M, Lisch D. Heritable transposon silencing initiated by a naturally occuring transposon inverted duplication. Nat Genet, 2005, 37(6) : 641-644. [31] Rudenko G N, Walbot V. Expression and posttranscriptional regulation of maize transposable element MuDR and its derivatives. Plant Cell, 2001, 13(3) : 553-570. [32] Lisch D, Chomet P, Freeling M. Genetic characterization of the Mutaor system in maize: behavior and regulation of Mu transposon in a minimal line. Genetics, 1995, 139(4) : 1777-1796. [33] Woodhouse M R, Freeling M, Lisch D. The mop1 (mediator of paramutation1) mutant progressively reactivates one of the two genes encoded by the MuDR transposon in maize. Genetics, 2006, 172(1) : 579-92. [34] Zhu Z G, Fu Y P, Xiao H, Hu G C, Si H M,Yu Y H, Sun Z X. Ac/Ds transposition activity in transgenic rice population and DNA flanking sequence of Ds insertion sites. Acta Bot Sin, 2001, 45(1) : 102-107. [35] Dinges J R, Colleoni C, Myers A M, James M G. Molecular structure of three mutations at the maize sugary1 locus and their allele-apecific phenotypic effects1. Plant Physiol, 2001, 125(3) : 1406-1418. [36] Girard L, Freeling M. Mutator-suppressible alleles of rough sheath1 and liguleless3 in maize reveal multiple mechanisms of suppression. Genetics, 2000, 154(1) : 437-446. [37] Chuck G, Meeley R B, Hake S. The control of maize spikelet meristem fate by the APETALA2-like gene indeterminate spikelet1. Genes Dev, 1998, 12(8) : 1145-1154. [38] Fowler J E, Muehlbauer G J, Freeling M. Mosaic analysis of the ligueless3 mutant phenotype in maize by coordinate suppression of Mutator-insertion alleles. Genetics, 1996, 143(1) : 489-503. [39] Greene B, Walko R, Hake S. Mutator insertion in an intron of the maize knotted1 gene result in dominant suppressible mutations. Genetics, 1994, 138(4) : 1275-1285. [40] McCarty D R, Carson C B, Stinard P S, Robertson D S. Molecular analysis of viviparous1: an abscisic acid-insensitive mutant of maize. Plant Cell, 1989, 1(5) : 523-532. [41] Chalvet F, Grimaldi C, Kaper F, Langin T, Daboussi M J. Hop, an active Mutator-like element in the genome of the fungus Fusarium oxysporum. Mol Biol Evol, 2003, 20(8) : 1362-1375. [42] Lisch D, Freeling M, Langham R J, Choy M Y. Mutator transposase is widespread in the grasses. Plant Physiol, 2001, 125(3) : 1293-1303. 遗传学报 Acta Genetica Sinica Vol.33 No.6 2006 [43] Rossi M, Araujo P G, de Jesus E M, Varani A M,van Sluys M A. Comparative analysis of Mutator-like transposases in sugarcane. Mol Gene Gen, 2004, 272(2) : 194-203. [44] Mao L, Wood T C, Yu Y, Budiman M A, Tomkins J, Woo S, Sasinowski M, Presting G, Frisch D, Goff S, Dean R A, Wing R A. Rice transposable elements: a survey of 73000 sequence tagged-connectors. Genome Res, 2000, 10(7) : 982-990. [45] Yoshida S, Tamaki K, Watanabe K, Fujino M,Nakamura C. A maize MuDR-like element expressed in rice callus subcultured with praline. Hereditas, 1998, 129(1) : 95-99. [46] Hudson M, Ringli C, Boylan M T, Quail P H. The FAR1 locus encodes a novel nuclear protein specific to phytochrome A signaling. Genes Dev, 1999, 13(15) : 2017-2027. [47] Wang H, Deng X W. Arabidopsis FHY3 defines a key phytochrome signaling component directly interacting with its homologous partner FAR1. EMBO J, 2002, 21(6) : 1339-1349. [48] Hudson M E, Lisch D R, Quail P H. The FHY3 and FAR1 genes encode transposes-related proteins involved in regulation of gene expression by the phytochrome A-signaling pathway. Plant J, 2003, 34(4) : 453-471. [49] Diao X M, Freeling M, Lisch D. Horizontal transfer of a plant transposon. Public Library of Sciences, Biology, 2006, 4(1) : 119-128. [50] Kellogg E A. Evolutionary history of the grasses. Plant Physiol, 2001, 125(3) : 1198-1205. [51] Lisch D. Pack-MULEs: theft on a massive scale. Bioessays, 2005, 27(4) : 353-355. [52] Bennetzen J L. Transposable elements, gene creation and genome rearrangement in flowering plants. Curr Opin Genet Dev, 2005, 15(6) : 621-627. [53] Fedoroff N. Transposons and genome evolution in plants. Proc Natl Acad Sci USA, 2000, 97(13) : 7002-7007. [54] Shapiro J A. Transposable elements as the key to a 21st century view of evolution. Genetica, 1999, 107(1-3) : 171-179. [55] Talbert L E, Chandler V L. Characterization of a highly conserved sequence related to Mutator transposable elements in maize. Mol Biol Evol, 1988, 5(5) : 519-529. [56] Yu Z, Wright S I, Bureau T E. Mutator-like elements in Arabidopsis thaliana: structure, diversity and evolution. Genetics, 2000, 156(4) : 2019-2031. DIAO Xian-Min et al.: Mutator Transposon in Maize and MULEs in the Plant Genome 487 Mutator 转座子及 MULE 在植物基因与基因组进化中的作用 刁现民 1,Damon Lisch2 1. 河北省农林科学院谷子研究所 国家谷子改良中心,石家庄 050031; 2. 美国伯克力加州大学植物与微生物学系,伯克力 CA 94720,美国 摘 要:Mutator(Mu)转座子是植物中已发现的转座最活跃的转座子,其高的转座频率及趋向于单拷贝功能基因转座的特 性,使该转座子成为玉米功能基因克隆的主要方法。Mu 转座子的同源类似因子广泛存在于被子植物基因组中,而且同一 基因组中往往具有多种变异类型。它不仅具有其他 DNA 转座子在基因和基因组进化中的普遍作用,而且具有能够承载基 因组内功能基因和基因片段的载体功能,这种载体 Mu 转座子(Pack-MuLEs)能够在基因组内移动众多的基因片段,从而 对基因和基因组进化产生作用。Mu 转座子的同源序列发生在水稻与狗尾草之间的水平转移提供了高等植物核基因水平转 移的首个例证。对 Mu 转座子的了解促进了我们对动态基因组概念的认识。文章对 Mutator 转座子的发现、转座特征、基 因标签应用等的研究进展进行了综述,对 Mu 转座子家族的同源序列进行了分类,讨论了该转座子在基因组进化中的作用, 分析了应加强研究的问题。 关键词:Mutator 转座子;基因组进化;MuLE;载体 Mu 转座子 作者简介:刁现民(1963-),男,河北南和县人,理学博士。研究方向:作物遗传育种与分子生物学