* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The supramammillary area: its organization, functions
Aging brain wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Neuroplasticity wikipedia , lookup
Memory consolidation wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Haemodynamic response wikipedia , lookup
Neural oscillation wikipedia , lookup
Theta model wikipedia , lookup
Apical dendrite wikipedia , lookup
Metastability in the brain wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Subventricular zone wikipedia , lookup
Development of the nervous system wikipedia , lookup
Adult neurogenesis wikipedia , lookup
De novo protein synthesis theory of memory formation wikipedia , lookup
Neuroanatomy wikipedia , lookup
Environmental enrichment wikipedia , lookup
Sexually dimorphic nucleus wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Hypothalamus wikipedia , lookup
Optogenetics wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Synaptic gating wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Progress in Neurobiology 74 (2004) 127–166 www.elsevier.com/locate/pneurobio The supramammillary area: its organization, functions and relationship to the hippocampus Wei-Xing Pana, Neil McNaughtonb,* a b Department of Physiology and Center for Neuroscience, University of Otago, POB56, Dunedin, New Zealand Department of Psychology and Center for Neuroscience, University of Otago, POB56, Dunedin, New Zealand Received 20 March 2004; accepted 15 September 2004 Abstract The supramammillary area of the hypothalamus, although small in size, can have profound modulatory effects on the hippocampal formation and related temporal cortex. It can control hippocampal plasticity and also has recently been shown to contain cells that determine the frequency of hippocampal rhythmical slow activity (theta rhythm). We review here its organization and anatomical connections providing an atlas and a new nomenclature. We then review its functions particularly in relation to its links with the hippocampus. Much of its control of behaviour and its differential activation by specific classes of stimuli is consistent with a tight relationship with the hippocampus. However, its ascending connections involve not only caudal areas of the cortex with close links to the hippocampus but also reciprocal connections with more rostral areas such as the infralimbic and anterior cingulate cortices. These latter areas appear to be the most rostral part of a network that, via the medial septum, hippocampus and lateral septum, is topographically mapped into the hypothalamus. The supramammillary area is thus diffusely connected with areas that control emotion and cognition and receives more topographically specific return information from areas that control cognition while also receiving ascending information from brain stem areas involved in emotion. We suggest that it is a key part of a network that recursively transforms information to achieve integration of cognitive and emotional aspects of goal-directed behaviour. # 2004 Elsevier Ltd. All rights reserved. Contents 1. Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 128 2. Anatomy of the supramammillary area . . . . . . . . . . . . . . . . . . . . . 2.1. The boundaries of the supramammillary area and its parts . . 2.1.1. Where is it best to place the boundary of SuM?. . . . 2.1.2. What are the parts of SuM?. . . . . . . . . . . . . . . . . . 2.1.3. Construction of an atlas of SuM. . . . . . . . . . . . . . . 2.2. The connections of SuM . . . . . . . . . . . . . . . . . . . . . . . . . . 2.2.1. Afferents to SuM . . . . . . . . . . . . . . . . . . . . . . . . . 2.2.2. Efferents from SuM . . . . . . . . . . . . . . . . . . . . . . . 2.2.3. Neuronal types in relation to projections from SuM . 2.2.4. Neuronal types in relation to projections to SuM . . . . . . . . . . . . . 129 129 130 132 133 138 138 140 140 140 3. Neurophysiology of SuM . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.1. SuM and hippocampal theta activity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.1.1. What is theta activity? . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 141 141 141 * Corresponding author. Tel.: +64 3 479 7634; fax: +64 3 479 8335. E-mail address: [email protected] (N. McNaughton). 0301-0082/$ – see front matter # 2004 Elsevier Ltd. All rights reserved. doi:10.1016/j.pneurobio.2004.09.003 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 128 W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 . . . . . . 141 143 146 147 147 148 4. Behaviour and c-fos activation of SuM . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 148 5. SuM 5.1. 5.2. 5.3. 5.4. 5.5. . . . . . . 150 150 150 151 152 152 6. SuM, behavioural change and theta frequency . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6.1. Theta frequency change and behaviour: relative change or absolute value? . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6.2. Changes in behaviour with no absolute change in theta frequency . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 153 153 155 7. SuM—an interface between cognition and emotion? . . . . . . . 7.1. SuM, behaviour and hippocampal function . . . . . . . . . 7.2. SuM, the amygdala and thalamus . . . . . . . . . . . . . . . . 7.3. SuM and forebrain control of emotion . . . . . . . . . . . . 7.4. SuM—a controller of cognitive–emotional interaction? . . . . . . 155 156 157 157 159 References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 159 3.2. 3.3. 3.1.2. The septum as a pacemaker for hippocampal theta activity . 3.1.3. Integration of theta frequency by SuM under urethane . . . . 3.1.4. Descending influences controlling SuM theta activity . . . . . 3.1.5. Multiple frequency integrators in freely moving animals . . . SuM and hippocampal modulation . . . . . . . . . . . . . . . . . . . . . . . . Multiple influences of SuM on the hippocampus . . . . . . . . . . . . . . dysfunction and behaviour. . . . . . . . . . . . . . . . Methods of inducing SuM dysfunction . . . . . . Spatial cognition . . . . . . . . . . . . . . . . . . . . . Exploratory and defensive behaviour . . . . . . . Behavioural inhibition. . . . . . . . . . . . . . . . . . SuM lesions compared to hippocampal lesions. . . . . . . . . . . . . . . . . . . 1. Introduction The supramammillary area (SuM) is a relatively thin, chemically distinct, layer of cells overlying the mammillary bodies in the hypothalamus. While relatively few in number, the cells of this area have recently become of interest as they appear to exert significant modulatory control over the hippocampus and, directly and via the hippocampus, substantial portions of the telencephalon. Thus, like the raphe serotonergic system and the coerulear noradrenergic system, SuM may have functional importance out of all proportion to its size. Its connection with the hippocampus is made early in primate development and it has been suggested that ‘‘the early ingrowth of the excitatory SuM–hippocampal system in human and non-human primates may contribute to the prenatal activity-dependent development of the hippocampal formation’’, perhaps as a result of its control of hippocampal theta activity (Berger et al., 2001). It contrasts with the mammillary bodies in being much more a source of input to archicortex (particularly hippocampus) than a receptor of output from it. It also contrasts, as far as can be told, with many other hypothalamic areas in being a specific controller of hippocampal theta activity. It is, then, located in an area of the brain usually associated with emotion and motivation and not only connects with areas of the brain thought to be involved in predominantly cognitive functions but controls a pattern of brain activity (theta) that has been postulated to be important for control of cognition and . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . particularly memory. We argue, on several different grounds, therefore, that its modulation of telencephalic activity is fundamental to cognitive–emotional interactions. This claim is subject to two caveats. First, we are claiming only a modulatory role for SuM. It is likely to pace information around circuits but not to encode and transform that information itself. It is likely to determine whether other inputs produce plasticity in target structures but not to induce plasticity solely through its own inputs. Second, as will become clear, it is likely to be only the first discovered of a number of (perhaps topographically organized) structures that function in this specific way. To both make our claim, and flesh out our caveats, we analyse the anatomy, physiology and contributions to behavioural function of SuM. We focus, in particular, on the extent to which SuM may discharge behaviourally important functions through its afferent and efferent connections with the hippocampal formation. Our conclusions may seem to present SuM as fundamentally controlling hippocampal function—but not only must SuM be only one of several structures that control the hippocampus (each, we argue under different behavioural or cognitive circumstances) but also the hippocampus must be only one of many widely separated structures modulated by SuM. Some of the parallels we identify between SuM and hippocampal function are likely, therefore, to be functions that depend on the joint activity of many ‘‘limbic’’ structures. W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 Our specific link with the hippocampus is made by the fact that SuM has recently been shown to contain cells that can control plasticity in the hippocampus via monosynaptic input. It also contains other cells that control the frequency of the rhythmic phasic firing of hippocampal cells (theta activity) via a relay in the medial septum (MS). These two types of interaction with the hippocampus are functionally separate and anatomically distinct. But they are of such a kind (controlling general tone and controlling the timing of firing of large populations of cells) that it is likely that SuM neurones play a much more important modulatory role in hippocampal function than their paucity and distance from the hippocampus might suggest. SuM also has similar extensive connections with many other structures and may, then, similarly modulate many areas of the forebrain. The hippocampus has been postulated to contribute to cognitive functions such as spatial mapping (O’Keefe and Nadel, 1978) and relational memory (Cohen and Eichenbaum, 1993) and emotional functions (Papez, 1937), particularly behavioural inhibition (Gray, 1982; Gray and McNaughton, 2000). All components of the hippocampal formation, including the entorhinal and posterior cingulate cortex as well as hippocampus proper (Gray and McNaughton, 2000; Leung and Borst, 1987), show theta activity. This can be viewed as regular phasic firing controlled by pacemaker impulses relayed to the hippocampal formation from the MS (Brucke et al., 1959; Gogolák et al., 1968; Petsche et al., 1962; Tömböl and Petsche, 1969). Thus, the time at which the population of cells in the hippocampus fires is strongly influenced by afferent theta activity that produces regular changes in membrane potential (Bland, 1986). This control could be viewed as septal input actively eliciting theta activity in the hippocampus. However, it is probably better viewed as a temporal gating of activity by phasic inhibitory input (Leung, 1998). Thus when cells are permitted to fire is determined largely by phasic release from inhibition (Smythe et al., 1992), but it is also influenced by other factors (O’Keefe and Recce, 1993; Bose and Recce, 2001). By contrast, which cells fire during the appointed time is determined by other specifically patterned inputs. Theta activity is likely to play a significant specific role in the cognitive and/or emotional functions of the hippocampal formation (O’Keefe and Nadel, 1978; Gray, 1982; Miller, 1991; Gray and McNaughton, 2000) as well, perhaps, as a more general role in the control of sensori-motor integration (Bland and Oddie, 2002; Oddie and Bland, 1998) or sensory inhibition (Sainsbury, 1998). The postulated power of the control of hippocampus (and other areas like entorhinal, posterior cingulate and possibly perirhinal cortex) by SuM rests in the possibility that a failure of appropriate timing of computations carried out between these structures could result in serious degradation or even total loss of the critical information (Miller, 1991). The frequency of hippocampal theta activity is controlled in part by medially placed SuM neurons (Kirk, 1993, 1997, 129 1998; Kirk and McNaughton, 1991, 1993). However, lateral rather than medial areas of SuM project preferentially to the MS (Vertes, 1992; Vertes and Kocsis, 1997; Vertes and McKenna, 2000). There is also monosynaptic input from more laterally placed SuM neurons (but not more medially placed ones) to the hippocampus. The ascending pathways that control theta and other hippocampal activity appear, then, to be topographically organized and to be both direct and relayed (as with the MS). The hippocampus, in turn, has topographically organised, relayed, projections back to the hypothalamus including SuM (Risold and Swanson, 1996). The details of the system, then, need to be understood if its function (and by implication that of much of the forebrain) is to be fully understood. SuM, at least on occasion, controls the frequency of theta activity. Theta activity, in turn, is most associated with the hippocampus. SuM is, therefore, likely to be important for the behavioural functions of the hippocampus. As noted, different parts of SuM may also contribute differentially to hippocampal function. However, as detailed below, SuM also has extensive connections with many other areas. We would expect it (through similar computational mechanisms) to contribute to a variety of different functions. This paper, therefore, reviews the neuroanatomy, neurophysiology and molecular biology of SuM, and provides a preliminary assessment of the functions of SuM, the relation of these functions to its known parts, and the extent to which those functions are discharged by interaction with the hippocampus and particularly by the control of theta activity by SuM. Abbreviations used in the remainder of the text are defined in Table 1 to allow ready reference. 2. Anatomy of the supramammillary area 2.1. The boundaries of the supramammillary area and its parts There are small but important differences in the definition and dissection into parts of SuM by different researchers (Fig. 1). We will focus below on a concordance between the full-scale atlases of Paxinos and Watson (1998) and Swanson (1998). According to the rat brain atlas of Paxinos and Watson (1998) (see Fig. 1), SuM is a small nucleus or set of nuclei, which is about 2000 mm 500 mm 500 mm, lying above the mammillary bodies and below the posterior hypothalamic area. The lateral area of the hypothalamus borders it dorsolaterally. The caudal lateral part of the area is named, by them, the lateral SuM (abbreviated by them, SuML), and the caudal medial part is named medial SuM (SuMM), but the rostral part is simply called SuM and is not explicitly divided into medial and lateral parts. It is unclear from this precisely how Paxinos and Watson separate the subnuclei within SuM. 130 W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 Table 1 Abbreviations used within the text (excluding figures) AMPA CCK CG DBB DR DRL FI GABA LS LH MS MB MR pm RPO SuM SuMc SuMg SuMl SuML SuMm SuMM SuMp SuMs SuMx (RS)-a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid CholeCystoKinin Central grey/periaqueductal grey Diagonal band of Broca Dorsal raphe Differential reinforcement of low rates of response: a schedule in which a reward can be obtained if the animal makes a response that is delayed by at least the DRL interval (often 15 s) since the last response, i.e. a reward would always be obtained if the animal responded every 15.1 s and would never be obtained if it responded every 14.9 s Fixed interval schedule: a schedule in which a reward can be obtained if the animal makes one response at any time after a fixed interval (often 60 s) has expired since the last reward was obtained Gamma amino butyric acid Lateral septum Lateral hypothalamus (no specific boundaries) Medial septum Mammillary bodies Median raphe Principal mammillary tract/mammillothalamic tract Nucleus reticularis pontis oralis Supramammillary area, largely as defined by Swanson (1998) including parts of the LH of Paxinos and Watson (1998)—essentially all those nuclei immediately superior to MB Supramammillary core—most central and compact portion of SuM, including SuMp and SuMg. see section at AP2.2, Fig. 4 Grandicellular supramammillary nucleus—contains but is not limited to distinctive large cells and is placed between SuMp and SuMs, see section at AP2.2, Fig. 4 Lateral supramammillary nucleus as defined by Swanson (1998) Lateral supramammillary nucleus as defined by Paxinos and Watson (1998) Medial supramammillary nucleus as defined by Swanson (1998) Medial supramammillary nucleus as defined by Paxinos and Watson (1998) Parvicellular supramammillary nucleus—most medial portion of SuM, see section at AP2.2, Fig. 4 Supramammillary shell—most lateral and diffuse portion of SuM, see section at AP2.2, Fig. 4 Nucleus of the supramammillary decussation—location as in Paxinos and Watson’s (1998) supramammillary decussation, boundaries of the nucleus poorly defined, see Section 2.2 Swanson (1998) divided SuM into a medial part (abbreviated by him, SuMm)1 and a lateral part (SuMl). The medial part contains smaller cells and the lateral part larger ones (Swanson, 1982; Risold and Swanson, 1997). This division was on the basis that (a) SuMm includes dopaminergic cells, which provide a major input to the lateral septal nucleus (LS), but not to the hippocampus (Swanson, 1982); while (b) SuMl contains large cells that project directly to the hippocampus (Haglund et al., 1984; Maglóczky et al., 1994) and the entorhinal area (Swanson, 1982). As we can see in Fig. 1, the differences between the two atlases are not only how they divide SuM into two parts, but also the shape of the nucleus and, in particular, the location of the lateral border of the SuM area. The SuM area in Swanson’s atlas is larger. Swanson’s SuMl includes parts of the lateral hypothalamus (LH) of Paxinos and Watson and, at its most rostral, includes their submammillothalamic nucleus. We must, therefore, answer two questions before proceeding to any analysis of the SuM area: (1) what is its boundary; (2) what is its division into subnuclei. 1 Conveniently, the two atlases use different abbreviations. So while ‘‘medial supramammillary nucleus’’ is ambiguous, SuMM is the medial part as defined by Paxinos and Watson while SuMm is the, different, medial part defined by Swanson. 2.1.1. Where is it best to place the boundary of SuM? Since we will later be subdividing SuM into distinct nuclei, the initial inclusion or exclusion of any particular homogenous area in our definition of SuM is to some extent arbitrary. We will concern ourselves first, therefore, with the boundaries between distinct nuclei above the mammillary bodies and only later consider whether they should be counted as parts of the more general supramammillary area (SuM). The location of the ventral boundary of SuM is largely uncontroversial. It can be taken, by exclusion, to be the most dorsal limits of the mammillary bodies and almost all the different authorities agree on the boundary of the latter. There are minor differences in the precise depth within the area between the principal mammillary tracts (pm) at which this boundary is placed. We have found that, with the neurotoxin AMPA, there is a very distinct boundary between totally lesioned supramammillary cells and virtually totally spared mammillary cells (see Fig. 2B and C) that is not seen with ibotenic acid lesions. This places the boundary almost exactly half way between the most dorsal and most ventral extent of the pm in sections taken at 4.5 mm posterior to bregma. This placement also corresponds to an area that can appear relatively clear of cells in normal histology (see Fig. 2A). This placement of the boundary is slightly higher than the position shown by Maglóczky et al. (1994), lower than W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 131 Fig. 1. Comparison of the boundaries of SuM as given in different sources. (A) Modified from Hayakawa et al. (1993). Superimposed on the drawings of SuM are dots representing terminals arriving from the preoptic area. These extend beyond the lateral boundary of SuM, as defined by the authors, in the most rostral section. (B) Modified from Maglóczky et al. (1994). Superimposed on the drawings of SuM are cells retrogradely labelled from the hippocampus. Note that these cells cluster around pm in the two more rostral sections and maintain an equivalent position more caudally. Note also the wider boundaries assigned to SuM laterally by these authors than in A. (C) From Vertes and McKenna (2000). (D) From the electronic copy of the atlas of Paxinos and Watson (1998). Note the limited lateral extent of SuM, extending only a small way beyond pm. (E) From the electronic copy of the atlas of Swanson (1998) with line thicknesses modified. Swanson (1982) and essentially the same as Borhegyi and Leranth (1997) and as Paxinos and Watson (1998). We will therefore take it as the ventral limit of SuM for the purposes of the present review. The dorsomedial boundary of SuM is also fairly well agreed on, running in an essentially flat line 300–500 mm above pm. Disagreement starts lateral to pm, at which point SuM is either represented as continuing in a straight line or in a descending curve, depending on the lateral limits that are accepted for SuM (see below). We turn now to a consideration of the lateral limits of SuM. These are quite different depending on whether one takes Paxinos and Watson or Swanson as a guide. Superficial inspection of thionin stained sections can support both types of division. As can be seen in Fig. 4 (section 4.5), the area immediately dorsal to the mammillary bodies includes a densely packed medial core and a more loosely packed lateral shell. The atlas of Paxinos and Watson allocates the rostral core to SuM but incorporates the shell into LH. Caudally they divide SuM into SuMM and SuML and this would be consistent with a similar subdivision of rostral SuM. Swanson’s atlas defines part of the core, which Fig. 2. Gross histological observations of SuM (Pan and McNaughton, 2002) and the effects of AMPA lesions on medial SuM (mSuM) and the mammillary bodies (MB). (A) A section taken from an unlesioned rat. Note that in this section there appears to be a thin cell free zone corresponding to the division between mSuM and MB. A similar separation was observed between lesioned and intact cells at this level. (B) A section taken from a more caudal level than (A) from an AMPA lesioned rat. The line marks the division between mSum and MB with cells above the line having been replaced by glia and cells below the line remaining intact. (C) Section from a second rat with a substantial AMPA lesion of mSuM demonstrating the lack of damage to MB as a whole. contains dopamine cells, as SuMm. He adds the remainder of the core to the shell to generate his SuMl. The question, then, is whether it is necessary to include the shell (Paxinos and Watson’s LH) with Paxinos and 132 W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 Watson’s SuML, treating it as a single area, Swanson’s SuMl. One reason for inclusion would be some structural or functional consistency between the shell and the core. The distributions of CCK-immunopositive cells (Lantos et al., 1995) and calretinin cells (Borhegyi and Leranth, 1997) clearly delineate the more medial portions of SuM and extends homogeneously into, and in parts delineates, the LH area of Paxinos and Watson. ‘‘The supramammillary nucleus [is] far more rich in CCK-ir perikarya than any other premammillary-posterior hypothalamic nuclei’’ (Lantos et al., 1995). There is a similar distribution of expression of 5HT1A receptor mRNA (Gundlah et al., 1999). These data suggest considerable chemical similarity between the core and the shell areas. Swanson (1982) also found that his SuMl (core and shell) as a whole projects directly to the entorhinal area and receives input from the caudal dorsal part of lateral septum whereas the core as a whole receives input from the caudal ventral part of lateral septum (Risold and Swanson, 1997). This projection distinguishes the shell (Swanson’s SuMl) from other parts of LH. Taken together, these criteria allow a convenient description of SuM itself as the entire area immediately superior to the mammillary bodies. As such it is an area that stains distinctly for CCK and calretinin, and that has its dorsolateral boundary defined by projections to the entorhinal area. So, in agreement with Swanson (1998) we will include a large medial portion of the LH of Paxinos and Watson within SuM for the purposes of this review. As will be seen, not only does this place ‘‘SuM’’ immediately above the mammillary bodies, the parts we can distinguish of SuM (so defined) appear to mirror the conventional mediolateral division of the mammillary bodies. 2.1.2. What are the parts of SuM? As we have seen there is good reason to follow Swanson and include more lateral areas in SuM. However, there are also good reasons for the division chosen by Paxinos and Watson. In particular, there is reason to distinguish the core from the shell, a division that separates Swanson’s SuMl into distinct parts. There is a high level of trkC mRNA expression in the core but not the shell (Numan and Seroogy, 1999). The cells projecting into the hippocampus, especially into dorsal hippocampus, are mainly located in the core, particularly around pm (Maglóczky et al., 1994; Haglund et al., 1984); although there are scattered cells in the shell. There are neurons clustered in the core, around the principal mammillary tract, which project to both MS/DBB and the hippocampus (Borhegyi et al., 1998). For all these reasons, it seems proper to treat the shell area as separate from the lateral part of the core. On similar grounds it is necessary to divide the core area into two parts. The core in the region that surrounds pm is distinct in containing large cells, having strong projections to the hippocampus and having cells that do not stain for substance P (Borhegyi and Leranth, 1997). Indeed, Paxinos and Watson classify the rostral part of Swanson’s SuMl as a quite distinct area, SMT. The more medial core region has small cells, has only weak connections to hippocampus and has cells that stain for substance P, including ones that are double labelled for substance P and calretinin. The substance P cells appear to be the source of the weak projection from the most medial portions of SuM to the hippocampus and they terminate on interneurones in the dentate gyrus and cells in CA2 and CA3 in the monkey (Leranth and Nitsch, 1994; Nitsch and Leranth, 1993) appearing early in prenatal development (Berger et al., 2001). The same projections are present in the rat, guinea pig and cat but mostly contain calretinin with substance P only present in the projection to area CA2 (Nitsch and Leranth, 1993; Borhegyi and Leranth, 1997; Gall and Selawski, 1984; Ino et al., 1988). The medial core region is also distinctive in containing a large number of dopaminergic neurones (Swanson, 1982; Gonzalo-Ruiz et al., 1992a,b). It seems best, therefore, to include in SuM all the area above the mammillary bodies that stains for CCK and calretinin. The dorsal and lateral boundaries of SuM we then take, given this inclusion, essentially as the consensus boundaries of the atlases. We then divide this overall area, where it is immediately above the mammillary bodies, into three major parts (Fig. 3). We leave open the possibility (see below) that there is a fourth part, above these three, located in the supramammillary decussation. Given previous nomenclature that uses the terms supramammillary, medial and lateral in differing and, often, ambiguous ways we are also proposing a new, nonoverlapping, nomenclature (with non-overlapping abbreviations) derived from the superficial appearance of the cells in the different areas. The most central area, we have termed the parvicellular SuM (SuMp). This is characterised most easily by having small cells (10–15 mm, Borhegyi and Leranth, 1997) and being centrally located without making contact with pm. As noted above, it is also histochemically distinct in containing cells that label for substance P and in having a substantial number of dopamine cells (Fig. 3). It corresponds to the most medial part of medial SuM in most other classifications. It is largely the same as Swanson’s SuMm except that its border remains clear of pm. The next area, moving laterally, we have termed the grandicellular SuM (SuMg). This is characterised most easily by the fact that it contains large cells (30 mm, Borhegyi and Leranth, 1997) that are clustered around, or located above and to either side of, pm. But these large cells are scattered among smaller cells and so the boundary of the nucleus does not correspond to the location of the large cells themselves. The nucleus corresponds, caudally, in its lateral boundary to Paxinos and Watson’s SuML and rostrally to their SMT. It is a medial portion of Swanson’s SuMl. It is histochemically distinct in containing cells that stain for caltretinin but not substance P. The next, and most lateral, area we have termed the supramammillary shell (SuMs). This corresponds to the lateral part of the SuMl of Swanson and to the ventromedial part of the LH of Paxinos and Watson. It is characterised by W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 Fig. 3. Lower part of figure: the parts of SuM in relation to its cell types and their connections to entorhinal cortex, septum and hippocampus. Cells are indicated by symbols indicating specific characteristic histochemical markers but with no indication of co-localisation of multiple markers. SuM as a whole contains calretinin-containing cells. The most medial portion of SuM contains small cells (and is therefore termed by us the parvicellular portion) and is distinguished by having substance P containing cells and a high density of dopamine containing cells. Around the pm fibre tract (solid black) is an area most clearly distinguished by containing large cells (and is therefore termed by us the grandicellular portion) that has projections to the medial septum as well as to the hippocampus. More laterally is an area with much more loosely packed cells (and so termed by us the shell portion) that is most clearly delineated by containing cells that project to entorhinal cortex. The parts of SuM as described by us occupy approximately the same medio-lateral positions as the parts of the mammillary bodies. Lateral to both SuM and the mammillary bodies, and occupying an intermediate dorso-ventral position are clusters of histaminergic cells that we see as bordering both the mammillary and supramammillary areas. Upper part of figure: interconnections of parts of SuM with the septum and hippocampus. Excitatory connections are indicated with solid lines and arrows and inhibitory ones with dashed lines and rectangles. The medial septum is divided into three distinct zones with an intermediate zone characterised by presumed cholinergic cells and an outer zone characterised by calretinin containing cells that receive projections from the entorhinal cortex (see text) (Borhegyi and Freund, 1998; Vertes and McKenna, 2000; Borhegyi et al., 1998; Kiss et al., 2002; Borhegyi and Leranth, 1997; Leranth et al., 1999; Leranth and Kiss, 1996; Maglóczky et al., 1994; Vertes, 1992; GonzaloRuiz et al., 1992). smaller cells that are less tightly packed than those of SuMp. It does not stain for trkB mRNA (Numan and Seroogy, 1999) but is otherwise like SuMg in projecting to the entorhinal area and staining for CCK (Lantos et al., 1995) 133 and calretinin (Borhegyi and Leranth, 1997). Functional divisions appear consistent with these morphological ones. The most marked increases in c-fos activity are in the core of SuM with little change in the shell with challenges such as changes in temperature (see Section 4). As to the boundary of the lateral side of the shell area, we follow Swanson’s atlas. This leaves a small amount of tissue lateral to it (but not above the mamillary bodies) as lateral hypothalamic area. This is populated with histaminergic cells. These latter were included in SuM as defined by Maglóczky et al. (1994) (see Fig. 1B). However, not only are these cells chemically distinct from SuM, their location at some levels is somewhat more ventral than is typical of the parts of SuM and nonetheless lateral to what is generally accepted as the mammillary bodies. The above description accounts for the area immediately above the mammillary bodies. However, there is a loose aggregation of cells located in the supramammillary decussation, immediately above SuMp, which also stain for CCK. This nucleus (if such a diffuse cluster can be treated as such) is not supramammillary in the same sense as SuMp, SuMg and SuMs since it is separated from the mammillary bodies by SuMp. However, given our use of CCK as a basis for including nuclei within SuM, it should probably be included for the sake of completeness within our nomenclature. It also appears functionally similar to SuMp in that both show high c-fos expression (relative to the number of cells in the area) in rats placed in an open field (Ito et al., 2003). The area (as opposed to the cells themselves) is also labelled, elsewhere, as the supramammillary decussation (usually abbreviated sumx). We think it reasonable, therefore, to refer to it as the nucleus of the supramammillary decussation (SuMx) both to avoid confusion from the earlier terminology and because the area shares with all the other supramammillary nuclei the staining for CCK that we have used to delineate them. However, since its conformation at the more rostral and caudal levels of the supramammillary area is not known, we have not included it in the consensus atlas described in the next section. Nor does it figure largely in the data reviewed later. 2.1.3. Construction of an atlas of SuM Our novel separation and delineation of the parts of SuM led us to construct an atlas of SuM as a means of providing concordance between and with other atlases and publications. In addition, the small size of SuM coupled with its detailed division suggested that a finer grain separation of successive sections would be advantageous. Our first step was to produce drawings that integrated the atlas of Paxinos and Watson with that of Swanson. To do this we traced over the drawings from Swanson’s atlas and overlaid these drawings on the corresponding nearest match in Paxinos and Watson to produce an approximation to a fixed number of tens of millimeters A-P from bregma. In some cases the same (intermediate) Swanson drawing was overlaid on two (anterior and posterior respectively) 134 W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 Fig. 4. An atlas of SuM. This shows consensus drawings derived from the atlases of Paxinos and Watson (1998) and Swanson (1998) as described in the text. Matching sections from one rat selected from our own histology are also shown. The division of SuM into its individual parts is described in detail in the text but can be thought of as accepting the two part divisions of both atlases to achieve a resulting three-part consensus. Where AP location is indicated in normal roman numerals the drawing is an average of the equivalent drawings of the two atlases. Where it is in italics the drawing is an average interpolated between two other drawings, except at 5.0 where it was derived from the matching histological section since the conformational change between the adjacent sections was too great for interpolation to be accurate. Note that the boundaries of the grandicellular portion of SuM (dashed lines) do not coincide with the, unclear, limits of the main occurrence of the large cells from which we have derived the name. The approximate location of the main concentration of large cells within SuMg is indicated by a change of texture. W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 Fig. 4. (Continued ). 135 136 W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 Fig. 4. (Continued ). W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 Fig. 4. (Continued ). 137 138 W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 drawings from Paxinos and Watson to generate successive cases. We then traced lines intermediate between those of the two atlases. Our second step was to fill the gaps at 4.3, 4.6, 4.8, and 5.0 mm by interpolation (Fig. 4). There was a remaining gap at 5.1 mm, which could not be bridged by interpolation since the change in form between 5.0 and 5.2 mm is too great. This gap was filled using our own histology after the next step described below. Our third step was to match the drawings with our own histological material (Fig. 4). From over 100 cases of histology, we selected a continuous series of 100 mm thick serial sections that corresponded in plane of section and general conformation to that of our atlas drawings. We first anchored these sections in relative AP location with respect to the drawings by the transition from the presence of the ventral tongue of the third ventricle at 4.4 mm to its absence at 4.5 mm. We then matched sections with drawings in simple 100 mm steps from this point rostrally and caudally. The correspondence between the remaining drawings and sections was assessed as a check on the accuracy of the interpolation of the drawings between the atlases and of the positional matching of the resultant drawing to our sections. No A-P adjustment was required and, once this was confirmed, the histological section at 5.1 mm was used as a basis for the drawing provided at this level. A few minor inconsistencies were detected at this point between particular drawings and our sections. These turned out to be due to anomalies in the equivalent drawing in one or another of the atlases both when that drawing was compared with the other atlas and when it was compared with the preceding and following drawing from the same atlas. These drawings were then brought into line with the preceding and following drawings, which also achieved correspondence with our histology. Our fourth step was to take SuMg, defined from the atlases as described above, and divide it roughly into two parts, one containing only smaller cells and the other containing both small and large cells. This is marked in the drawings by a change in pattern within the line bounding the nucleus but, since there is no strict boundary between the two parts, they are not separated by a solid or dotted line, unlike the major divisions of SuM. As noted above, we have not included SuMx in the atlas as its conformation, or indeed existence, in more rostral and caudal sections is not well defined in the literature. 2.2. The connections of SuM SuM is one of a range of brainstem areas that project to the hippocampus (Wyss et al., 1979; Segal and Landis, 1974; Pasquier and Reinoso-Suarez, 1978a,b; Amaral and Cowan, 1980; Stanfield and Cowan, 1984; Stanfield et al., 1980)— The others include the raphe nuclei and locus coeruleus. Like the monoamine nuclei, despite its small size, SuM produces direct diverse innervation of many forebrain areas. This suggests that SuM, like the monoamine systems, may execute some general modulatory, possibly emotional, function. We have suggested elsewhere that theta activity (which is controlled by SuM) may produce an improvement in the signal–noise ratio of neuronal processing in relation to temporal summation that is equivalent to the improvement in signal–noise ratio produced by the monoamine systems in relation to spatial summation (Gray and McNaughton, 2000). Consistent with a role in emotion, SuM connects with widespread regions of the limbic system, such as anterior limbic cortex, amygdala, entorhinal cortex, dentate gyrus, septum, preoptic area, anterior hypothalamus, LH, thalamus, locus coeruleus, DR and MR (Swanson, 1976, 1982; Shibata, 1987; Vertes, 1992; Hayakawa et al., 1993; Thinschmidt, 1993; Risold and Swanson, 1997; Vertes et al., 1999; Swanson and Cowan, 1975, 1979; Conrad and Pfaff, 1976; Saper et al., 1976; Krayniak et al., 1980; Ottersen, 1980; Sakanaka et al., 1980; Contestabile and Flumerfelt, 1981; Price and Amaral, 1981; Deacon et al., 1983; Kiyama et al., 1984a,b; Haglund et al., 1984; Chiba and Murata, 1985; Shibata et al., 1986; Grove, 1988). That its role involves higher (more rostral/cortical) levels of the nervous system is suggested by the fact that its connections avoid the striatum and the nucleus accumbens (Swanson, 1982; Vertes, 1992). Fig. 5 illustrates the main connections of SuM taken as a whole. There is some semblance of a pattern in these connections. SuM is reciprocally connected with more rostral, essentially, limbic cortical and subcortical areas. It has predominantly efferent connections to somewhat more caudal, again essentially limbic (and thalamic), areas. Where its connections are predominantly afferent, they are subcortical and, except for the habenula, from relatively low subcortical levels. In Fig. 5, there are a few structures that on current data appear to depart from the expectation of reciprocal connections. These are the dorsal hypothalamus, the ventromedial hypothalamus and the premammillary area. 2.2.1. Afferents to SuM The main ascending afferents are of two types. There are inputs that are likely to be modulatory from the cholinergic laterodosal tegmental nucleus, and the serotonergic raphe (Gonzalo-Ruiz et al., 1999). There are also inputs that are likely to convey specific information or be involved in specific functional output from the median raphe, and the CG (Gonzalo-Ruiz et al., 1999; Shibata, 1987; Vertes et al., 1999; Hayakawa et al., 1993). There is no solid evidence that the magnocellular RPO and the pedunculopontine tegmental nucleus in the brain stem project directly into SuM (Hayakawa et al., 1993; Thinschmidt, 1993). One report of a connection between RPO and SuM (Vertes and Martin, 1988) appears to be due to the absorption of the tracer by fibres of passage (Hayakawa et al., 1993). This lack is surprising since, in W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 139 Fig. 5. General connections of SuM ignoring its division into parts. The figure is an exploded diagram approximating a 3D representation and has been derived from the 2D representation in Paxinos and Watson (1983; Fig. 78–80) with the addition of known monosynaptic connections (Vertes, 1988, 1992; Hayakawa et al., 1993; Shibata, 1987; Risold and Swanson, 1997; Swanson, 1982; Thinschmidt, 1993; Kiss et al., 2002). Dashed lines with arrows indicate connections currently thought to be unidirectional, solid lines with balls indicate those known to be bidirectional. There appears to be a topographic division of ascending connections with bidirectional connections going to more ventral and rostral areas and unidirectional ones going to more dorsal and caudal areas (DH, VMH and PM appear to be exceptions). The representation of LHb has been displaced caudally to achieve clarity and to locate it closer to the superior colliculus (the area above CG), CG and DR with all of which it has connections. Abbreviations: AH, anterior hypothalamic area; AM, anteromedial thalamic nucleus; Amyg, amygdala; AV, anteroventral thalamic nucleus; Ce, cerebellar nuclei; CG, central gray; CgCx, cingulate cortex; CM, central medial thalamic nucleus; DBB, diagonal band of Broca; DH, dorsal hypothalamic area; DP, dorsal peduncular cortex; DR, dorsal raphe nucleus; EC, entorhinal cortex; Hip, hippocampus proper; IL, infralimbic cortex; IP, interpeduncular nucleus; L6, spinal cord, lamina 6; LC, locus coeruleus; LDTg, laterodorsal tegmental nucleus; LH, lateral hypothalamus; LHb, lateral habenula; LS, lateral septal nucleus; MB, mammillary bodies MD, mediodorsal thalamic nucleus; mfb, medial forebrain bundle; MR, median raphe nucleus; MS, medial septal nucleus; PH, posterior hypothalamic area; PM, premammillary nucleus; PO, preoptic nuclei; Re, nucleus reuniens; SI, substantia innominata; SuM, supramammillary area; VMH, ventromedial hypothalamus; VTg, ventral tegmental nucleus. urethane-anaethsetised animals, electrical stimulation of these areas elicits theta very effectively (Vertes, 1982) and, under these conditions, SuM appears to act as the sole relay to the hippocampus (Kirk and McNaughton, 1993). The main descending afferents to SuM are from the infralimbic cortex, the dorsal peduncular cortex, DBB, the medial and lateral septal nuclei, the lateral habenula and the medial and lateral preoptic area (Vertes, 1988, 1992; Hayakawa et al., 1993; Shibata, 1987; Risold and Swanson, 1997; Swanson, 1982; Thinschmidt, 1993; Chiba and Murata, 1985; Kiss et al., 2002). In this respect the connections of SuM appear similar to those of the mammillary bodies and in many cases are likely to be collaterals of the same neurones (Hayakawa and Zyo, 1994). It should be noted, however, that hippocampal output is topographically mapped in such a way that information destined for SuM arises in area CA3 and is first relayed in the lateral septum, while information destined for MB arises in the subiculum and is transferred direct to SuM (Risold and Swanson, 1996). The inputs to the dopaminergic cells of SuMp have been suggested to be excitatory (Hayakawa and Zyo, 1994). GABAergic neurons located at the border between the LS and MS project to SuM (Borhegyi and Freund, 1998; Leranth et al., 1999). These cells contain calretinin and/or calbindin. They form a skin that surrounds choline acetylase containing cells. These in turn surround ‘‘like the skins of an onion’’ (Borhegyi and Freund, 1998) GABAergic parvalbumin-containing neurones (Kiss et al., 1997a,b; Henderson et al., 2001). These centrally located GABAergic cells also have projections to SuM—but these are very weak compared to the cells on the MS/LS border and the cells in LS (Fig. 3). The calretinin containing cells do not project to the hippocampus or the amygdala (Kiss et al., 1997a,b). The various types of medial septal cell appear to be highly interconnected via recurrent axon collaterals (Henderson et al., 2001; Kiss et al., 1997a,b). It was previously thought that LS cells might also control MS cells (Leranth and Frotscher, 1989). However, more modern methods suggest that LS–MS connections are sparse with the main hippocampal feedback being direct to MS and strong inhibitory connections from MS to LS (Leranth et al., 1992). The LS/MS/DBB projections to SuM are likely to be the basis for complex interrelations with the hippocampal formation and related structures. The GABAergic cells on the border of MS/LS relay information from the entorhinal cortex to SuM (Leranth et al., 1999), but their precise topographic organisation is not known. However, the 140 W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 hippocampal formation as a whole is topographically mapped into the lateral septum and this in turn is topographically mapped into the hypothalamus (Risold and Swanson, 1996) including SuM (Borhegyi and Freund, 1998). This suggests that for some functions, SuM is a relatively small part of a much larger parallel system. 2.2.2. Efferents from SuM SuM sends ascending projections to the diencephalon and telencephalon (Swanson, 1976, 1982; Shibata, 1987; Vertes, 1992; Hayakawa et al., 1993; Thinschmidt, 1993; Risold and Swanson, 1997; Vertes et al., 1999; Swanson and Cowan, 1975, 1979; Conrad and Pfaff, 1976; Saper et al., 1976; Krayniak et al., 1980; Ottersen, 1980; Sakanaka et al., 1980; Contestabile and Flumerfelt, 1981; Price and Amaral, 1981; Deacon et al., 1983; Kiyama et al., 1984a,b; Haglund et al., 1984; Chiba and Murata, 1985; Shibata et al., 1986; Grove, 1988). SuMg and SuMs project to the MS, the DBB, hippocampus, entorhinal cortex, cingulate cortex, frontal cortex, and amygdala. SuM as a whole, projects to LS, the preoptic area, most of the medial nuclei of the thalamus, the subthalamus and other parts of the hypothalamus (for references see Fig. 5). The descending projections from SuM (principally from SuMg and SuMs) are mainly to the CG, MR, DR and locus coeruleus (for references see Fig. 5), and even the cerebellar nuclei in monkeys (Haines et al., 1990) and spinal cord in rabbits (Gong, 1984). On a large scale, the projections from SuM tend to show a simple topographic organisation. The medial SuM mainly projects to more medial structures and the lateral SuM mainly projects to more lateral structures. For instance, the dopamine cells of SuMp project to the LS, the large cells of SuMg project to the hippocampus and the small cells of SuMg and SuMs project to the entorhinal cortex. Similarly, SuMp projects mainly to the medial preoptic area and SuMs project mainly to the lateral preoptic area. 2.2.3. Neuronal types in relation to projections from SuM SuM neurons include dopaminergic cells (Swanson, 1982; Gonzalo-Ruiz et al., 1992a,b), calretinin neurons, aspartergic/glutamatergic cells (Carnes et al., 1990; Leranth and Kiss, 1996; Kocsis et al., 2003), substance P neurons as shown in Fig. 3 (Ino et al., 1988; Gall and Selawski, 1984; Borhegyi and Leranth, 1997), and CCK immunoreactive neurons (Kiyama et al., 1984a,b; Lantos et al., 1995) and VIP immunoreactive neurons (Haglund et al., 1984; Seroogy et al., 1988). The dopaminergic cells are located mainly in SuMp (Fig. 3), and project to the LS (Swanson, 1982; Shepard et al., 1988) and mamillary bodies (Gonzalo-Ruiz et al., 1992a,b). Swanson (1982) thought that the dopaminergic cells in SuM could be a dense extension of the A10 (ventral tegmental area) cell group. Shepard et al. (1988) argued that those cells do not appear to be a rostral extension of the A10 neurons since the cytological characteristics of the dopaminergic cells in SuM differ from the cells in A10. The former are significantly smaller than the latter (Phillipson, 1979; Shepard et al., 1988). It is well known that both the dopaminergic pathway from A9 to the striatum and the one from A10 to the nucleus accumbens play a role in reward learning. There are no direct data implicating the SuM–LS or SuM–MB dopaminergic pathway in reward. But, singleunit recording has shown that the firing of the dopaminergic cells of SuM shows essentially the same response pattern to conditioned stimuli as the dopaminergic cells of A9 and A10 (Pan and Hyland, 2001). Likewise (Ikemoto et al., 2003), the cholinergic agonist carbachol, when self-administered into VTA, produces c-fos expression in SuM neurons, which suggests that SuM may be linked to the reward system. Calretinin containing neurons are distributed throughout SuM (Borhegyi and Leranth, 1997). Some of the calretinin neurons are aspartergic/glutamatergic neurons. These are mainly in SuMg and SuMs, and project to MS cholinergic cells and LS calbindin cells (Leranth and Kiss, 1996) and hippocampus (Kiss et al., 2000). In contrast, the glutamatergic neurons located in SuMp project to the medial preoptic area (Kocsis et al., 2003). Some calretinin neurons (Borhegyi and Leranth, 1997), substance P neurons (Borhegyi and Leranth, 1997; Gall and Selawski, 1984; Ino et al., 1988), and VIP immunoreactive neurons (Haglund et al., 1984) project to the hippocampus. In rats, substance P fibres from SuMp terminate only in the CA2 region and, in contrast to more lateral areas of SuM, not in the dentate gyrus (Borhegyi and Leranth, 1997). In monkeys, however, they contact dentate granule cells, hilar cells and CA3 cells (Leranth and Nitsch, 1994) and are formed prenatally (Berger et al., 2001). These latter projections are present in the rat but contain calretinin and not substance P. In rats, CCK immunoreactive neurons project to the nucleus anterior ventralis thalami (Kiyama et al., 1984a,b), ventral tegemental nucleus (Kiyama et al., 1984a,b), and the hippocampaus (Greenwood et al., 1981). Cells in SuMg that project to the hippocampus also contain estrogen receptors. These receptors appear to be responsible for plastic changes in the density of spine synapses in area CA1 that result from changes in systemic estrogen level (Leranth and Shanabrough, 2001). The postsynaptic targets of SuM projections in the hippocampus are both principal cells (Maglóczky et al., 1994) and GABAergic interneurons (Nitsch and Leranth, 1996). These neural circuits could all play a role in the control of hippocampal theta rhythm. 2.2.4. Neuronal types in relation to projections to SuM Combining retrograde labelling with immunohistochemistry techniques, Gonzalo-Ruiz et al. (1999) mapped the localization of neurotransmitter-related molecules in the main projection neurons to the SuM. They found that there are 1. Cholinergic neurons projecting from the MS/DBB and the laterodorsal tegmental nucleus to the SuM, and they suggested these connections were likely to be excitatory. W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 2. GABA-containing neurons projecting from the compact subnucleus of the central superior nucleus and from DBB to the SuM, which they suggested are likely to be inhibitory. 3. Serotonergic neurons projecting to the SuM from DR. 4. A few neurons projecting to the SuM from the rostroventral part of the subiculum, the DBB and the DR containing glutamate-like immunoreactivity, which they suggested are likely to be excitatory. 5. Neurons containing neuropeptides projecting to the SuM which are from several sources—somatostatin-positive neurons from the medial preoptic nucleus, enkephalinpositive neurons from the compact subnucleus of the central superior nucleus, neurotensin-positive neurons from the medial preoptic nucleus, DR and compact subnucleus of the central superior nucleus, and substance P neurons from the laterodorsal tegmental nucleus. In addition, Leranth et al. (1999) showed that the GABAergic neurons located in the border between the LS and MS (but not within the main body of these nuclei) project to calretinin containing (non-GABAergic) cells in SuM. These data show that the ascending and descending projections to the SuM are chemically very diverse. They are likely, therefore, to be the basis of complex interactions in SuM. 3. Neurophysiology of SuM 3.1. SuM and hippocampal theta activity 3.1.1. What is theta activity? Hippocampal ‘‘theta rhythm’’ (Jung and Kornmüller, 1938; Green and Arduini, 1954) is one aspect of hippocampal EEG, which in some species is within the human EEG theta range (4–8 Hz) but in the rat and some other species can span frequencies between 4 and 14 Hz. Hippocampal theta rhythm recorded extracellularly with gross electrodes results from sources and sinks associated with the synchronised oscillations of IPSPs or EPSPs, or perhaps both (Fujita and Sato, 1964; Nunez et al., 1987, 1990; Leung, 1998). These also determine the phasic firing of hippocampal neurons (Bland, 1986). We will term the phasic firing of individual neurons ‘‘theta activity’’. When this theta activity (synchronized phasic firing of individual cells) is present in individual neurons it tends to involve the entire hippocampal formation. But in some areas, such as CA3, large-scale synchronous firing of individual neurons is not accompanied by a gross recordable rhythm. The frequency and amplitude of theta rhythm have been the main measures of the frequency and population distribution, respectively, of single cell theta activity in the majority of studies. The synchronous firing of hippocampal cells, and the frequency of the resultant theta activity is critically dependent upon the constant inflow of impulses from 141 outside the hippocampus. The MS and DBB are known to be the last relay in the ascending synchronising inputs to the hippocampal formation. MS/DBB cells act as the ‘‘pacemaker’’ for hippocampal theta. Each burst of septal cell firing produces synchronous changes in the excitability of the hippocampal formation as a whole (Gogolák et al., 1968; Petsche and Stumpf, 1960; Petsche et al., 1962; Sailer and Stumpf, 1957; Stumpf, 1965; Stumpf et al., 1962; Chrobak et al., 1987, 2000; Lee et al., 1994). The pathways controlling theta frequency appear to originate in the brain stem reticular formation in the region of the pons (Vertes, 1981, 1982, 1986; Vertes and Kocsis, 1997). These sites of origin are well caudal to the areas known to project directly to SuM (Fig. 5) and SuM is well caudal to MS. Probably one of the most important facts about the electrophysiology of the core of SuM is that it contributes to the control of the frequency of hippocampal theta activity and that, at least on some occasions, the MS appears to be simply a relay for supramammillary activity. 3.1.2. The septum as a pacemaker for hippocampal theta activity2 The immediate source of the high degree of concurrent synchrony not only across the entire hippocampal formation but also the entorhinal and posterior cingulate cortices, is the MS/DBB complex. The MS/DBB is best thought of, in this context, as a pure ‘pacemaker’. The term ‘pacemaker’ is to some extent ambiguous. Its simplest meaning (and the one we will use here) is that it is a structure that controls phasic activity, ‘‘pacing’’ the firing of its target cells. When training a runner, it is often convenient to have someone who runs ahead for some period to ‘‘set the pace’’. In this sense the frequency of hippocampal theta is controlled by the septal pacemaker since phasic firing of the hippocampus simply follows that of the septum and so has the same pace. However, ‘‘pacemaker’’ could also be taken to mean the site at which the phasic frequency of both structures is itself calculated by the integration of non-phasic inputs. As we will see there is at present no evidence that the MS/DBB is a pacemaker in this sense and considerable evidence against it. To use our running analogy, the pacemaker controls the speed at which the followers run—but it is the coach who will have determined what pace that should be (and who may alter it from time to time by instructions). In the brain, the structure in which non-phasic inputs are integrated and converted into frequency of phasic output will also be a ‘‘pacemaker’’ for the structures it controls. But we will reserve for it the term ‘‘frequency integrator’’ to distinguish it from the more passive pacemakers that relay alreadyphasic activity. Let us first consider the evidence for the septum as a simple pacemaker for hippocampal theta. As we noted above, there are cells in the MS/DBB which fire in bursts that are locked to a specific phase (for any individual cell) of the waves of the theta rhythm 2 This section is based on Appendix 5 of Gray and McNaughton (2000). 142 W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 simultaneously recorded in the hippocampus (Brucke et al., 1959; Petsche and Stumpf, 1960; Petsche et al., 1962; Gogolák et al., 1967, 1968; Tömböl and Petsche, 1969; Stumpf, 1965; Apostol and Creutzfeldt, 1974). GABAergic interneurons within the septum appear to keep the whole septum synchronized (Brazhnik and Fox, 1997). To simplify somewhat, phasic GABAergic input from the septum appears to combine with effectively tonic cholinergic input from the septum to control hippocampal phasic activity (Lee et al., 1994; Gray and McNaughton, 2000). Phasic activity in the septum could be the result of hippocampal input rather than vice-versa. However, destruction of the MS/DBB produces permanent disruption of hippocampal theta activity (Green and Arduini, 1954; Petsche and Stumpf, 1960; Stumpf, 1965; Gray, 1971; Rawlins et al., 1979; Bland et al., 1996). Medial septal procaine also eliminates both theta rhythm and theta activity in the entorhinal cortex (Dickson et al., 1995; Jeffery et al., 1995). Critically, injection of GABA agonists also reduces theta amplitude, without changing its frequency (Lamour et al., 1984; Dutar et al., 1989; Bland et al., 1996; Jiang and Khanna, 2004). This shows that GABA receptors in the septum are located on cells that transmit phasic activity to the hippocampus and that the effects of lesions and procaine are not due to an action simply on fibres of passage. By contrast, disruption of input from the hippocampus to the septum increases the regularity and persistence of theta activity in the septum (Vinogradova, 1995); disruption of hippocampal theta by medial septal lesions increases cingulate theta (Borst et al., 1987); and partial section of the descending columns of the fornix increases the frequency of theta, an effect which can be reversed with the noradrenergic agonist clonidine (Ammassari-Teule et al., 1991). These observations make sense if there is negative feedback control of the frequency integrator that controls (or is) the septal pacemaker for hippocampal rhythmicity. (The effects of medial septal lesions on cingulate theta can be understood if the MS/DBB pacemaker has a similar topographic organization to the MS/ DBB cholinergic system, with output to the cingulate cortex being from more ventral locations.). The effects of lesioning the descending columns of the fornix would tend to suggest that the site on which the negative feedback operates is the hypothalamus or some structure at a similar level of the brain. If the MS/DBB is a pacemaker is it also a frequency integrator? Here, temporary disruption of theta by injections of local anaesthetic into MS/DBB and surrounding regions is of particular interest. Injections of local anaesthetic reduce theta amplitude progressively even to the point of total elimination with no reduction in frequency (Dickson et al., 1995; Jeffery et al., 1995; Jiang and Khanna, 2004; Oddie et al., 1996). At intermediate doses, some of this local anaesthetic would be expected to affect input fibres as well as cell bodies and output fibres. We will discuss frequency integration in more detail later—but we would expect a reduction in input to the integrator to produce a reduction in theta frequency. The lack of such a frequency reduction with local anaesthetics argues against the septum normally acting as an integrator (but see also below). Similarly, injections of procaine into the medial forebrain bundle, which contains the input to the MS/DBB pacemaker, reduce the amplitude of theta to zero without affecting frequency (Kirk and McNaughton, 1993). This is in contrast to the effects of similar injections into SuM (Kirk and McNaughton, 1993)—which we will discuss further below. What kind of injection into a frequency integrator would be expected to reduce theta frequency? The frequency of theta varies linearly with the level of high-frequency (usually 100 Hz) input from reticular regions whether this level is altered by changing stimulation voltage/current, altering stimulating pulse width or altering the frequency of stimulation. This frequency is reduced by drugs that act on the GABA–benzodiazepine–chloride–ionophore complex to potentiate the effects of GABA, such as benzodiazepines and barbiturates (McNaughton and Sedgwick, 1978). The simplest explanation of these data is that the incoming level of tonic input is integrated as summed EPSPs in cells that then fire and are shortly shut down by recurrent GABAergic inhibition. Higher levels of input would overcome this recurrent inhibition sooner giving rise to a higher frequency (shorter interburst interval) of phasic output. Drugs that potentiate endogenously released GABA would decrease theta frequency. Drugs that act simply as tonic GABA agonists would tend to shut the system down altogether. We noted earlier that medial septal GABA has little effect on theta frequency. Unfortunately benzodiazepine injections have not been tested there. However, injections of barbiturate into the medial septum also do not reduce theta frequency even at concentrations that totally eliminate it (McNaughton, 1977)—and this elimination appeared to be due simply to the pH of the solution as it could be reproduced by simple sodium salts. These data all suggest that the MS/DBB contains a pacemaker for hippocampal theta activity but not, at least usually, a frequency integrator. Evidence for the septum as a pacemaker can also be drawn from the effects of electrical stimulation. Stimulation of the MS/DBB at theta frequencies drives hippocampal theta rhythm with each septal pulse producing a phaselocked theta wave (Ball and Gray, 1971; Gray and Ball, 1970; James et al., 1977; Wetzel et al., 1977a,b). However, spontaneous theta is replaced by small irregular activity if the MS/DBB is stimulated continuously at high frequencies, above about 70 Hz (Stumpf, 1965; Ball and Gray, 1971). This is what we could expect from blockade of the normal phasic pattern of MS/DBB firing. It would be difficult to understand if theta was the result only of tonic cholinergic afferent drive onto intrinsically oscillatory hippocampal neurons, or if the MS/DBB complex were the site of frequency integration. But it can be explained by there being both excitatory and inhibitory inputs from the MS/DBB to the hippocampal formation (Bland and Colom, 1993) and by the driving of the septum by phasic input from elsewhere. W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 The blocking of theta by high-frequency stimulation of MS/DBB is in strong contrast to the effects of stimulation of more caudal areas of the brain. Particularly in the pons, continuous high-frequency stimulation elicits theta, the frequency of theta then generally increasing with the strength of stimulation (Sailer and Stumpf, 1957; Stumpf, 1965; McNaughton and Sedgwick, 1978; Vinogradova, 1995). The transition between theta elicitation and theta blocking by high-frequency stimulation can, then, be used to indicate the location of the frequency integrator. In general, sites at which high-frequency stimulation elicits theta are well caudal to the MS/DBB and, critically, do not include sites in the medial forebrain bundle afferents to MS/DBB. Although found in many parts of the brain, including the lateral hypothalamus, the posterior hypothalamus and the midbrain reticular formation (Stumpf, 1965; Anchel and Lindsley, 1972; Whishaw et al., 1972; Robinson and Vanderwolf, 1978; Smythe et al., 1991) effective sites have not generally been reported rostral to SuM. Consistent with this, Vertes’s mapping of the ascending synchronizing pathway originating in nucleus reticularis pontis oralis leads to and stops at SuM (Vertes, 1980, 1981, 1982). There are a few apparent exceptions to this rule (Destrade, 1982; Destrade and Ott, 1982) with highfrequency stimulation of the medial forebrain bundle. However, these authors used trains with phasic gaps. This type of stimulation of the septum is known to be capable of driving theta in the same way as phasic single pulses (James et al., 1977), and inspection of their data (e.g. Fig. 1A and especially 1B in Destrade and Ott (1982)) suggests that their stimulation was driving theta at various harmonics of the gap frequency. According to the authors, the frequency of theta increased with increasing strength of stimulation, as would be expected with more posterior stimulation sites. However, inspection of the figures suggests that this was the result of increasing phase locking of activity with the gaps in the phasic delivery of the high-frequency pulses. Unlike conventional ‘theta driving’, the phasic frequency was below the theta range. However, septal input or sensory stimuli (Givens, 1996) can reset ongoing theta as well as drive it. Since the hippocampus has a tendency to intrinsic oscillation in the theta frequency range, Destrade’s results appear to be due to driving of various harmonics of the imposed phasic frequency, with the resultant theta frequency determined by the natural period of oscillation of the septohippocampal system. Thus, the driving in Fig. 1B of Destrade and Ott (1982) is at the third harmonic of the stimulation gap frequency. It is clear from the above that MS/DBB controls hippocampal theta activity in the sense of being a pacemaker. However it is also seems clear, contrary to earlier views, that it cannot normally be the site at which the intensity of ascending reticular activity is integrated into the frequency of theta activity (but see below). 143 3.1.3. Integration of theta frequency by SuM under urethane Given the data on MS/DBB, a search had to be made for an extra-septal frequency controller for hippocampal theta.3 This search led to the core of SuM. Vertes (1986) proposed, from mainly anatomical data, that SuM might act as a relay of brainstem input to the MS. Kirk and McNaughton (1991, 1993) refined this idea further and proposed that SuM rather than the MS/DB integrates the frequency of theta, at least in urethane-anaesthetised rats. They found that SuMp and SuMg cells discharge rhythmically and that they do so in phase with any concurrent hippocampal theta activity (Kirk and McNaughton, 1991). As far as can be told, all SuMp cells show theta activity as do cells in the most medial portions of SuMg (Kirk and McNaughton, 1991; Kirk et al., 1996; Kocsis and Vertes, 1994; Bland et al., 1995; Bland and Oddie, 1998; Kirk, 1998) and, under urethane, SuM generates a local theta rhythm with its phase varying relative to the hippocampus as frequency changes but with a fixed time delay of about 70 ms (Kocsis and Vertes, 1997; Kirk, 1997). Sampling of SuMg has been sparse but suggests that this may contain both rhythmic and non-rhythmic cells with, perhaps, the former located more medially (Kirk and McNaughton, 1991; Kocsis and Vertes, 1994). Alternatively, the lateral boundary between primarily rhythmic and primarily arhythmic populations of cells may be at the border between SuMg and SuMs (Kocsis and Vertes, 1994). The vertical boundary appears to be between SuMp and SuMx with cells in SuMx firing tonically rather than phasically (Bland et al., 1995). Kirk and McNaughton (1991) found that procaine injections into MS blocked hippocampal theta but not the rhythmic bursting of SuM cells (Fig. 6), suggesting that SuM cells controlled hippocampal cells rather than the other way round. Consistent with this, Kocsis and Vertes (1994) found that SuM firing rates were relatively homogenous in contrast ‘‘with the discharge of theta-related cells in other structures of the brainstem or forebrain’’. Kirk and McNaughton (1993) found (Fig. 7) that procaine injections into SuMp or SuMg decreased both the frequency and amplitude of reticular-elicited theta in urethaneanaesthetised rats, while procaine injected into the medial forebrain bundle decreased the amplitude only, and procaine injected into the reticular formation decreased the frequency only (Kirk and McNaughton, 1993; Kirk, 1993; Bland and Oddie, 1998). This shows that non-phasic reticular input enters SuM and is there integrated into phasic theta activity that leaves SuM. So Kirk and McNaughton (1993) proposed that, at least under urethane, the core of SuM integrates level of tonic reticular input to the frequency of theta output. As discussed above this integration is simply explained if the 3 Kirk and McNaughton (1993) used procaine mapping to locate SuM but then had to demonstrate SuM rythmicity (Kirk and McNaughton, 1991) before their results were accepted for publication. 144 W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 Fig. 6. Examples of unit activity in SuM and its relation to hippocampal theta. (A) One 240 ms sweep showing a burst of unit activity. (B–F) Sweeps of 2 s showing (top trace) hippocampal EEG activity and (bottom trace) unit activity from medial SuM. (B) Rhythmicity of SuM locked to hippocampal theta—this was shown by all cells. (C) Lack of rhythmicity of SuM during large irregular activity in the hippocampus (same electrode placement as (B)). (D) Rhythmicity of SuM retained after blockade of hippocampal theta by medial septal procaine injection (same placement as (B) and (C)). (E) A different site showing an example of relatively poor rhythmicity. (F) The same site as (E) showing improved rhythmicity after blockade of hippocampal theta with septal procaine. From Kirk and McNaughton (1991). frequency of theta output is determined by relatively fixed amounts of recurrent inhibition and higher levels of excitatory input overcome this, temporally decaying, inhibition at earlier timepoints. That is, output from principal SuM cells instantly activates recurrent inhibitory interneurones and so terminates the firing of both sets of neurons. As the resultant IPSPs decay over time, the level of inhibition is eventually overcome by the current level of afferent excitation, the cells pass threshold and fire a new burst of action potentials that, in turn, shut the system down again. It should be emphasised here that the ascending theta system is polysynaptic. Its source, in these experiments, is RPO (Vertes, 1980, 1981; Vertes and Kocsis, 1997). Yet, as noted earlier, this has no direct projection to SuM (Hayakawa et al., 1993; Thinschmidt, 1993). Likewise, we as well as others (Vertes and Kocsis, 1997; Bland and Oddie, 1998) have reason to see SuM as the source of phasic impulses that control the MS/DBB. Yet SuMp (the principle area targeted by Kirk and McNaughton) projects primarily to the LS not MS, while SuMg and SuMs project to the medial septum (Fig. 3). This suggests that any phasic control of theta activity by SuMp (which shows the clearest theta activity) is likely to be relayed, and SuMg is one likely candidate. However, anatomical evidence of a projection between SuMp and SuMg is lacking. The scattered cells in SuMp that do project to MS (Vertes, 1988) could perhaps provide all the necessary control. Alternatively they may be an extension into SuMp of a class of cells mostly located in SuMg. If so control of phasically firing MS cells would be distributed throughout the core of SuM. Recently, Jiang and Khanna (2004) replicated the effects of local anaesthetic found by Kirk and McNaughton (1993) using smaller injection volumes (0.1–0.2 mL). They obtained effects within SuMp but also in sites in SuMg and in the medial forebrain bundle dorsal to SuMg. Of particular interest, they also injected the inhibitory transmitter GABA. Previous injections of synaptically active agents into SuM have involved benzodiazepines. These would act on the benzodiazepine–GABA–chloride– ionophore complex to potentiate the effects of endogenously released GABA (Haefely, 1990a,b, 1991, 1992). A critical aspect of benzodiazepine function is that the benzodiazepine has no intrinsic inhibitory effect. It solely potentiates the effect of endogenous GABA. In the theta frequency integrator (assuming this depends on recurrent GABAergic inhibition), the benzodiazepines would increase the inhibitory power of the pulsed release of GABA from interneurones. However, as the GABA was eliminated from the synaptic cleft, the benzodiazepines would become ineffective and cell firing could recommence, The benzodiazepine would thus increase the duration of effect of endogenous phasically released GABA but without any permanent effect on excitability and so it would increase the interburst interval and so decrease the theta frequency of burst firing of cells but without any general reduction in cell firing (McNaughton et al., 1995). However, tonically applied GABA would remain in the synaptic cleft over tens of minutes rather than, like the benzodiazepine, potentiating the effects of endogenous GABA that would remain in the synaptic cleft only for tens of milliseconds. It would be expected therefore to depress firing generally and have little effect on the duration of action of endogenously released GABA. Consistent with this prediction, Jiang and Khanna found that GABA injected into SuMp reduced the amplitude of theta, ultimately eliminating it without reducing frequency. Their most significant finding was that GABA injections also had this effect when injected W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 145 Fig. 7. Effects on hippocampal theta of injection of 0.5 mL procaine at different sites before and at indicated times after injection. (A) Effect of injection into the medial septum. Note the almost immediate reduction in the amplitude of theta with, as shown by fast Fourier transform (FFT) no decrease in frequency. (B) Mapping of effective and ineffective sites. The boxed areas were mapped and symbols indicate sites at which there was an effect. Rostral to SuM injections reduced amplitude but not frequency (~) as illustrated in (A). Caudal to SuM injections reduced frequency but not amplitude (&) as illustrated in (C). In the region of SuM both effects were obtained together (*). (C) Effect of injection into the midbrain, caudal to SuM. Note the substantial change in frequency with minimal changes in amplitude. Adapted from Kirk and McNaughton (1993). into SuMg. There are also indications that effects in SuMg as well as SuMp can be produced in freely moving animals by injections of muscimol (Saji et al., 2000). The probability is, then, that the frequency of theta depends in part on integration (summation) of the inputs to SuMp cells (Kirk and McNaughton, 1991, 1993; Kirk, 1993; McNaughton et al., 1995; Kocsis and Vertes, 1997; Vertes and Kocsis, 1997; Bland et al., 1994; Kirk et al., 1996). But both the anatomy and the effects of GABA suggest that phasic activity could then be relayed laterally to SuMg, which then sends its output rostrally into the medial forebrain bundle and thence to the MS. Alternatively, injections of procaine or benzodiazepine, nominally into SuMp, might affect both it and SuMg with the SuMg cells 146 W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 that project to the septum being the key location of frequency integration. However, an exclusive involvement of SuMg seems impossible given the tight localisation of effects that can be demonstrated within SuMp (McNaughton et al., 1995). The most obvious frequency reductions produced by the benzodiazepines (either by direct injection or implied by injection of the antagonist after i.p. injection of the benzodiazepine) are in SuMp. However procaine injections directly into SuMg alter frequency as well as amplitude. If SuMp were the sole site of frequency integration, one might expect that SuMg procaine would reduce amplitude but not frequency—as is observed with injections into more rostral sites such as the medial forebrain bundle. A frequency reducing effect of procaine in SuMg could be due to an action on fibres of passage from more caudal areas passing through SuMg on their way to SuMp. But there are a wide variety of cell types intermingled in SuM. So it is possible that, while SuMp and SuMg can be distinguished by their containing distinct groups of cells unrelated to theta rhythm, that they have in common a single type of thetacontrolling cells (possibly calretinin positive but not substance P positive). These theta cells could then be evenly distributed across what we have classified as SuMp and SuMg. This suggestion is reinforced by data (Ito et al., 2003), reviewed in Section 4, that suggests that fos reactivity under conditions which activate hippocampus, is very strong in SuMp but also present in SuMg and comes from calretinin cells rather than, e.g. substance P cells. There may, then, be distinct non-theta-related populations of cells (substance P in SuMp and large cells in SuMg) embedded in a homogenous group of theta-related cells (calretinin positive spread through SuMp and SuMg). If this is true, however, it is difficult to see why the projection from SuMp to MS should be so weak relative to that from SuMg and why no non-rhythmic cells have been found in SuMp (Kirk and McNaughton, 1991). Further work, in particular extensive mapping of SuMg with benzodiazepines, is required to answer these questions. SuM cells innervate both cholinergic and GABAergic cells in MS/DBB (Borhegyi et al., 1998). These GABAergic and cholinergic cells then innervate hippocampal cells and are very likely to play a crucial role in theta rhythm (Frotscher and Leranth, 1985; Lee et al., 1994; Freund and Antal, 1988; Tóth et al., 1997; Smythe et al., 1992). The cells in SuM are, despite their consistent firing rates, of different types (as would be expected from its histochemical complexity, see Sections 2.2.3 and 2.2.4). Four distinct groups of cells fire at different phases of theta (Kocsis and Vertes, 1997). Those firing in phase and 1808 out of phase are likely to represent excitatory and inhibitory neurons and to act as a lower level of the parallel cholinergic and GABAergic systems (Kocsis and Vertes, 1997). This is not to say that there is a cholinergic projection from SuM to the medial septum. But there is reason to think that there are cholinoceptive cells in SuM (whatever transmitter they release in the septum) that contribute to the generation of theta rhythm (Oddie et al., 1994). These cholinoceptive cells are likely to receive their input from the cholinergic neurons projecting from the MS/DBB and the laterodosal tegmental nucleus to SuM. Under urethane, then, theta rhythm appears to arise when tonic activation of the reticular formation (particularly RPO) is relayed through unknown nuclei to SuMp (and perhaps SuMg) where it is integrated into phasic activity that is relayed via SuMg to MS/DBB and thence to the hippocampal formation. Consistent with this, under urethane, phasic firing is not observed in RPO whereas tonic theta-on cells are (Hanada et al., 1999). The generation of phasic activity is likely to depend on both excitatory cells and recurrent inhibitory interneurones, firing with different phase relations to each other (Kocsis and Vertes, 1997; Vertes and Kocsis, 1997). However, there are two ways in which we must complicate this already fairly complex picture. First, there are descending influences that may be involved in controlling the phase of theta or its amplitude (without necessarily affecting frequency). Second, in freely moving animals, other nuclei than the supramammillary nucleus can help to control theta frequency. 3.1.4. Descending influences controlling SuM theta activity That there are descending influences, even in urethaneanaesthetised animals, is shown by the fact that procaine injection into the medial septum, that abolishes hippocampal theta, reduces the number of spikes per burst of some SuMp cells. However, this change in firing pattern was obtained without any change in interburst interval and so without any change in the frequency of theta (Kirk and McNaughton, 1991). This finding suggests the loss of an excitatory or disinhibitory influence. Consistent with this, cells in SuMx that fire tonically rather than phasically (Bland et al., 1995) and become silent when hippocampus is showing theta may act to ‘‘inhibit discharge of theta-on cells in the posterior hypothalamus and SuM during non-theta hippocampal states’’ (Bland et al., 1995). However, procaine injection into MS that blocks theta but not the rhythmic bursting of SuM cells has also been reported to increase the number of spikes per burst (Kirk and McNaughton, 1991) or to increase the theta burst frequency of SuM cells (Kirk et al., 1996) (increased burst frequency being a decrease in the interburst interval). This suggests that hippocampal or septal output can inhibit SuM cells (Kirk et al., 1996). It seems likely that these different reported results may stem from sampling of different cell types. Kirk and McNaughton (1991) injected urethane i.p. and elicited theta with a tail pinch (or allowed it to occur spontaneously) in Sprague-Dawley rats. They reported that their SuM cells fired on a single phase of hippocampal theta matching just one of the four phase groups described by Kocsis and Vertes (1997) mentioned in the previous section. By contrast, Kirk W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 et al. (1996) injected urethane through a jugular cannula and elicited theta with RPO stimulation in Long-Evans rats. These plus other possible consistent differences (such as level of anaesthesia) could have resulted in them sampling different cells, or the same cells in a different state, than Kirk and McNaughton. The four distinct groups of SuM cells that fire at different phases of theta (Kocsis and Vertes, 1997) could then reflect both excitatory and inhibitory cells that send output to the medial septum (as already suggested above) and also excitatory and inhibitory cells receiving input from the hippocampus and related theta-generating structures. For example, entorhinal aspartate/glutamatergic cells innervate GABAergic neurons located at the border between the LS and MS that, in turn, project to calretinin positive SuMg cells (Leranth et al., 1999). These SuMg cells are known to project to MS (Vertes, 1992), hippocampus (Nitsch and Leranth, 1996) and so, closing the loop, entorhinal cortex (Swanson, 1982). This descending GABAergic pathway is likely to exercise an inhibitory influence on SuM. There may also be a descending excitatory pathway. High doses of carbachol injected into RPO replaced theta with sharp waves in the hippocampus and SuM and transection rostral to SuM returned it to theta activity (Kirk, 1997). Matching the ascending parallel excitatory and inhibitory pathways controlling theta, there may then be parallel descending excitatory and inhibitory pathways. Under conditions where these do not influence frequency of theta as such (Kirk and McNaughton, 1991) it is likely that they are nonetheless either involved in the control of amplitude or are part of the phase reset system (Givens, 1995). 3.1.5. Multiple frequency integrators in freely moving animals That SuMp is not the only area controlling theta in freely moving animals is shown by the fact that large lesions of SuM in freely moving rats did not affect the theta rhythm accompanying spontaneous movement (Thinschmidt et al., 1995). Nor do such lesions have large effects on reticularelicited theta (McNaughton et al., 1995). However, even specific SuM dysfunction produced with either benzodiazepine infusion or chemical lesion can produce modest decreases in theta frequency (by about 0.4 Hz) in some behavioural schedules such as FI and DRL (see Table 1). In these, the critical factor seems to be that the amount of movement is either not increased extensively by the treatment or is limited (e.g. by stereotypical execution) at the points in time when theta is assessed (Pan and McNaughton, 1997, 2002). With an FI schedule, injection of chlordiazepoxide into SuM can affect theta and behaviour as extensively as when it is injected i.p. (Woodnorth and McNaughton, 2002a,b) and injection of the benzodiazepine antagonist, flumazenil, can completely block the i.p. effects of the drug (Woodnorth and McNaughton, 2002a,b). 147 These data suggest that SuM may determine the frequency of even reticular-elicited theta (i) completely in urethane-anaesthetised rats, but (ii) only partly in freemoving rats. Similarly, SuM may control the frequency of theta under some behavioural conditions but not others and cooperate with other structures in controlling theta in yet further conditions. A complicating factor here is that movement elicits theta and that SuM lesions can increase movement. Where the absolute contribution of SuM to theta frequency appears modest in behaving rats it may then have a greater contribution relative to the specific level of the animal’s movement. Stimulation of the SuM area can produce limb stepping in anaesthetised rats (Sinnamon, 1984) but changes in theta were not assessed in this paper. Movement–theta relations need, therefore, to be very carefully controlled in future experiments. Despite this quantitative caveat, SuM clearly cannot be the only nucleus controlling theta frequency since theta activity can be essentially unaffected and never totally disappears in freely moving rats after even large SuM lesions that would be expected to destroy the bulk of input fibres even when they do not destroy all SuM cells (Thinschmidt et al., 1995; McNaughton et al., 1995). Capsaicin, a neurotoxin that selectively damages unmyelinated primary sensory neurons, also hits the SuM core (Ritter and Dinh, 1988), which suggests that the core of SuM might mediate sensory input. This would be consistent with an involvement of SuM in the controlling of theta that is elicited by tail pinch or reticular stimulation rather than by movement. There are reports of subcortical areas different from SuM that contain cells showing theta activity (Kocsis et al., 2001; Kocsis and Vertes, 1992, 1996) or even theta rhythm (Routtenberg and Taub, 1973). But, unfortunately, none of these have been challenged with septal blockade of hippocampal theta and could easily be driven by hippocampal output rather than acting as hippocampal input. It should also be noted that, given our hypothesis of theta frequency integration through recurrent inhibition, both the MS/DBB and the hippocampus itself (which contain the appropriate circuits) have the capacity for intrinsic oscillation and could on occasion act as frequency integrators. However, at present, there is no evidence that they ever do so under normal conditions (as opposed to when, e.g., superfused with carbachol). 3.2. SuM and hippocampal modulation SuM stimulation has been reported to have a potent inhibitory effect on cells in the dentate gyrus with the remarkably short latency of 3–5 ms (Segal, 1979) but also has been reported to potentiate population spikes in the dentate gyrus elicited by perforant path stimulation (Mizumori et al., 1989; Nakanishi et al., 2001; Carre and Harley, 1991). This apparent contradiction was resolved in part by evidence that SuM stimulation that increased population spikes was inhibiting inhibitory interneurones 148 W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 (Mizumori et al., 1989). However, it should also be noted that stimulation of noradrenergic or serotonergic fibres that inhibit ongoing spontaneous single-unit activity also potentiate population spikes, essentially because they increase signal to noise ratio (Segal, 1977; Segal and Bloom, 1974, 1976; Assaf, 1978; Assaf et al., 1979; Assaf and Miller, 1978). It is possible, therefore, that SuM exerts a similar influence, suppressing low levels of activity but enhancing higher levels. An important point to note, given the involvement of SuMp in the control of theta frequency is that effective stimulation sites for hippocampal potentiation were in SuMg. Sites in SuMp are ineffective. Potentiation from SuM or MS can be blocked by lesion of the dorsal fornix and potentiation from SuM can be blocked by lesions of the ascending columns of the fornix. However, critically, MS lesions do not block potentiation from SuMg (Mizumori et al., 1989). This suggests that potentiation arising in SuMg is transferred directly to the hippocampus and is unrelated to the pathways controlling theta frequency. Although SuMp does not appear to be involved in population spike modulation, a more general disinhibitory role for it has also been suggested by the fact that its inactivation can interrupt hippocampal epileptic discharge (Saji et al., 2000). While SuMg stimulation can potentiate hippocampal responses, stimulation in the region of RPO, at sites that can also elicit hippocampal theta, suppresses hippocampal population responses recorded in area CA1. Injections of procaine into SuMp or SuMg or MS attenuate this suppression (Jiang and Khanna, 2004). While it could be that RPO stimulation was activating cells in SuMg that both suppress CA1 responses and potentiate dentate responses this seems unlikely since, as noted above, medial septal blockade did not affect the latter whereas it does affect the former. The effects of RPO stimulation on theta can also be separated from its effects on CA1 suppression (Jiang and Khanna, 2004). First, suppression is produced at stimulation intensities that do not elicit theta and effects on theta increase steadily with changes in intensity that produce constant, maximal, suppression. Secondly, the time course of the effects of neuronal inactivation with procaine on the two responses is different. Thirdly, injections of GABA affect theta when they are made in both SuMp and SuMg but they only affect suppression of CA1 responses in SuMp. 3.3. Multiple influences of SuM on the hippocampus We have evidence, then, for multiple direct and indirect projections between SuM and the hippocampus. First, there are the neurones that control dentate gyrus potentiation. These are most likely to be one of the several cell types located in SuMg but could perhaps also involve the direct inputs from SuMp to the hippocampus that terminate on CA2, CA3 and interneurones of the dentate gyrus. Next, there are the neurones that control CA1 depression. These appear to have their cell bodies located in SuMp but then send their fibres laterally to pass through SuMg before joining the medial forebrain bundle. Finally, there are the neurones that control theta rhythm. These appear to be of several types. First, there are cells in SuMp (and possible SuMg) that determine the frequency of theta rhythm. These are likely to be the start of an ascending GABAergic inhibitory system that controls the timing of theta activity. However, they do not project directly to the GABAergic cells of the MS and it appears likely that cells in SuMg relay their output. Certainly (Jiang and Khanna, 2004) there are cells in SuMg that can control the amplitude of hippocampal theta activity and it seems likely, therefore, that two systems ascend from SuMg to control theta, one relaying with the GABAergic cells and one relaying with the cholinergic cells that together can control theta activity (Lee et al., 1994). 4. Behaviour and c-fos activation of SuM Activation, as shown by c-fos immunoreactive cell mapping, is found in SuM neurons in response to either electrical stimulation or microinfusion of the excitatory amino acid kainate or of the GABA antagonist SR-95531, applied to the medial hypothalamus—a defensive area (Silveira et al., 1995). Electrical stimulation of the dorsal CG, another defensive area, resulted in striking c-fos immunoreactivity in SuM (Sandner et al., 1992). SuM is also one area (others, Fig. 8, include the piriform and entorhinal cortices, amygdala, CG and DR) in which fos immunoreactivity was induced by 15 min exposure of rats to the elevated plus-maze, an ethologically based animal model of anxiety that involves no explicitly noxious stimulation (Silveira et al., 1993). Placing rats in a novel open field, which is also a task used to assess animal anxiety, showed that fos-like immunoreactivity was significantly more common in SuM cells projecting to the hippocampus than in those projecting to the midbrain (Wirtshafter et al., 1998). Recent data further show that the strongest reactivity induced by exposure to a novel open field is in SuMp (Ito et al., 2003). SuM is also activated in animals trained to run a radial arm maze when the maze is placed in a novel room (Vann et al., 2000). Interestingly, in the open field experiment (Ito et al., 2003) there was a high level of fos reactivity in the dentate and CA3 (as well as CA1) which, in the rat, receive input from calretinin positive cells of SuMp but not in area CA2, which is, in the rat, the only area receiving input from substance P containing SuMp neurons. Consistent with this, the fos reactivity, while much the strongest in SuMp was also present in SuMg which also contains calretinin cells. There may, then, be distinct functional populations of cells (substance P in SuMp and large cells in SuMg) embedded in a homogenous group of cells (calretinin positive in SuMp and SuMg). W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 149 Fig. 8. Areas showing c-fos reactivity in response to exposure to an elevated plus maze. There is clear selective activation of the supramammillary area in contrast to more lateral hypothalamic areas and to the mammillary bodies. More anterior portions of the hypothalamus also show reactivity as do parts of the superior colliculus, thalamus, central gray, amygdala, and ventrolateral portions of the cortex (adapted from Silveira et al. (1993)). SuM also is one of the only two structures in the brain (the other is retrosplenial cortex) which show a decrease in fearinduced c-fos expression with low dose diazepam (Beck and Fibiger, 1995). It is important to note, here, that the procedure they used was contextual rather than simple CS fear conditioning and that the diazepam produced a doserelated decrease in freezing to the context. This is consistent with the involvement of SuM in contextual fear conditioning described below. These data suggest that the core of SuM plays a role in defensive function and in the actions of anxiolytic drugs. In the open field, at least, the shell does not appear to be involved (Ito et al., 2003). SuM also expresses fos protein after cold or warm ambient exposure (Kiyohara et al., 1995; Miyata et al., 1995) or swim stress (Cullinan et al., 1996). More recently, a fos study (Lin et al., 1998) showed clearly that SuM is also one of the areas in which a significant number of fos-positive neurons in parturient and lactating rats was observed as compared to virgin or pregnant female rats. This activity may be in the cells that contain estrogen receptors and control spine synapse density in the hippocampus (Leranth and Shanabrough, 2001). The number of c-fos labelled neurons also increased in the core of SuM, especially in SuMp, after intravenous injection of naloxone (an opioid antagonist)—and the endogenous opioid system is thought to be involved in cardiorespiratory function and emotional and learning processes (Gestreau et al., 2000). All these data suggest that SuM may play a role in physiological stress and/ or emotional stress. Consistent with this, the areas of increased fos activity are almost all structures of the limbic system. However, despite its being conventionally seen as a critical node of the limbic system, the hippocampus appears silent. This is all the more surprising, given the demonstrated functional involvement of the hippocampus in behavioural tests that demonstrate SuM c-fos activation, such as the open field (Harley and Martin, 1999; Hausheer-Zarmakupi et al., 1996; Herman et al., 1998; Rossi-Arnaud and Ammassari-Teule, 1992), and given the involvement of the hippocampus in the control of stress hormones (Bhatnagar et al., 1997; Born and Fehm, 1998; Brake et al., 1999; Ferrini et al., 1999; Herman et al., 1998; Uno et al., 1989). There is also activation of SuM in the water maze during reference and working memory forms of the task (Santin et al., 2003). There will, of course, be effects of swim stress in these rats (Cullinan et al., 1996), but the increases in trained rats were relative to controls that received equivalent swimming time in the water maze. Interestingly, there was somewhat greater activation in the working memory than in the reference memory form of the task. There is also activation of SuM by exposure to an eight-arm (as opposed to one arm) radial maze condition (Vann et al., 2000). 150 W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 Surprisingly, given the above results, ventral tegmental injections of carbachol that induce c-fos expression throughout the brain, presumably related to the rewarding effects of the injections, seem to have a special effect in relation to SuM. Significant correlations between locomotion and c-fos-positive nuclei were found in the retrosplenial area and the posterior hypothalamus including the supramammillary nucleus. The authors focus on the retrosplenial area and supramammillary nucleus as areas that may, therefore, be parts of the circuitry for the reward triggered by ventral tegmental cholinergic stimulation (Ikemoto et al., 2003). This would not seem unreasonable given the presence of dopaminergic cells in SuM—but there is no other behavioural evidence for a link between SuM and reward. 5. SuM dysfunction and behaviour The electrophysiological data (both from theta and from analysis of hippocampal evoked potentials) suggest links between both SuMp and SuMg and the modulation of hippocampal processing. SuMs projects to the entorhinal cortex which is one of the major inputs to the hippocampus proper. These data all suggest that lesions of SuM should affect some behaviours in the same way as hippocampal lesions. Similarly, the c-fos data suggest that SuM is involved in emotional behaviour, particularly defensive behaviour, and that it is involved in at least some aspects of anxiolytic drug action. While the hippocampus is most often seen as being involved in memory rather than emotion, it should be noted that there are many apparently emotional tests (e.g. contextual fear conditioning) where hippocampal lesions have significant effects. The hippocampal formation has also been suggested to be the core of a ‘‘behavioural inhibition system’’ that mediates anxiolytic drug action (Gray, 1970, 1976, 1982; Gray and McNaughton, 2000). Both for some cognitive and some emotional tasks there are reasons (direct or indirect) to expect some similarity between the effects of SuM lesions and hippocampal lesions. However, SuM also has major descending connections with lower levels of the defense system and ascending connections with a multitude of limbic and extralimbic structures—although not being included in what some might see as the systems most fundamentally controlling defense (Risold and Swanson, 1997). A question for the current section, therefore, is: ‘‘to what extent do SuM lesions have effects specifically related to the non-hippocampal connections of SuM’’? These nonhippocampal connections, although they have not been electrophysiologically characterised, appear anatomically to be at least as important as the hippocampal ones. 5.1. Methods of inducing SuM dysfunction Given the location of SuM, close to the medial forebrain bundle and mammillary peduncles, specific conclusions can only be drawn with fibre sparing lesions. Likewise, given its depth in the brain, damage to more dorsal structures needs to be minimised. Pan and McNaughton (2002) developed an appropriate ‘‘minimal lesion’’ technique, using silica capillary tubing (140 mm external diameter) as an injection needle to minimize damage to the SuM, adjacent structures and all structures in the path of the needle. They compared the effects of the two popular neurotoxins—ibotenic acid and AMPA. They found that both ibotenic acid and AMPA injected into SuM destroyed both SuM and adjacent neurons. However, importantly, AMPA had no effect on the cells in the mammillary bodies (Fig. 2). With this latter technique, and small injections, it was possible to directly investigate, for the first time, the effect of fairly large selective lesions of SuM on behaviour. An alternative technique that has proved successful is to inject a benzodiazepine or GABA into SuM. Benzodiazepines reduce the frequency of hippocampal theta when injected systemically (McNaughton and Sedgwick, 1978; McNaughton and Coop, 1991) or directly into SuM (McNaughton et al., 1995). At least in urethane-anaesthetised rats, GABA injected into SuM can reduce the amplitude of theta and block it but without reducing frequency (Jiang and Khanna, 2004). It should be clear from the complex anatomy of SuM and from these simple electrophysiological results that it is important to record concurrent hippocampal theta activity when using these treatments. The treatment may reduce theta frequency or amplitude selectively and could also, clearly, have major effects on some parts of SuM without altering theta at all. The situation, as will be seen, is further complicated by the fact that whether SuM is involved in the control of theta depends on the behavioural state of the animal. 5.2. Spatial cognition The Morris water maze (Morris, 1984) is perhaps the quintessential test of hippocampal function (Morris et al., 1982). A key point is that it is a test of active rather than passive avoidance—a class of learning that is not normally affected by hippocampal lesions (Gray and McNaughton, 1983). It can be viewed, then, as a test of cognitive aspects of hippocampal function rather than of defensive systems. Spatial working memory can be tested in a single-day of continuous training in the water maze task (or assessed by within day changes with small numbers of trials). Spatial reference memory can be tested with multiple days of testing. With four trials per day for 4 days, systemic benzodiazepine treatment (5 mg/kg, i.p. chlordiazepoxide prior to each day’s testing), which might be expected to reduce theta frequency, impairs learning (McNaughton and Morris, 1987). By contrast, with the same behavioural testing quite large (albeit incomplete) lesions of the core of SuM (made well prior to training) decrease theta frequency only W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 modestly (by about 0.4 Hz) and produce little effect on spatial reference memory or swimming speed. Lesions of SuMs4 did not impair learning or theta (Pan and McNaughton, 2002). With multiple trials (and so complete learning) within 1 day, systemic benzodiazepine produces a large impairment in spatial learning and concurrent with this relatively large impairment it produces a reduction in theta frequency of about 1 Hz (Pan and McNaughton, 1997). By contrast, benzodiazepine infusion into SuM, decreased theta frequency by only 0.35 Hz in the first two trials and, at this time, produced no obvious learning impairment. A learning impairment did appear about the seventh trial. There could be, here, a partial confound. The rats were exposed to water for over 10 min and all rats in the experiment showed a logarithmic decrease in theta frequency over the course of training. Pre-exposure to water both decreases theta frequency (Whishaw and Vanderwolf, 1971; Pan and McNaughton, 1997) and impairs water maze learning (Pan and McNaughton, 1997). While the injection of benzodiazepine into SuM produced a modest behavioural effect in the late trials of this experiment, it also produced more of a reduction in theta frequency at this time when the effect of the drug might be expected to be wearing off. Primary treatment-induced deficits may then be potentiated by decreases in body temperature. SuM does not appear, therefore, to play a major role in either spatial working memory or reference memory or, indeed the theta that accompanies these tasks. However, the data are consistent with the idea that the small effect on theta frequency in early trials may have impacted on immediate consolidation (rather than working or reference memory as such) and so have affected later trials. If so, this would be consistent with effects on passive avoidance discussed below. This conclusion is consistent with the bulk of results from electrical lesion of SuM and the mammillary bodies in the radial arm maze (Jarrard et al., 1984). Large lesions of the mammillary bodies, SuM and surrounding tissue (including the ventral tegmental area and premammillary nuclei) do not affect visual–spatial conditional associative learning (Sziklas et al., 1995) although such lesions do impair radial arm maze learning. Smaller lesions, still including the medial mammillary bodies and SuM, do not affect radial arm maze learning (Sziklas and Petrides, 1993, 1998; Sziklas et al., 1996). An important point, when we consider later tests, is that the data show only that SuM is minimally involved in both the control of behaviour and theta in the water maze. Theta activity itself (controlled by some other area) is involved in spatial learning, since systemic benzodiazepine or cooling by water exposure both decreased theta frequency by about 1 Hz (to lower then 6.5 Hz) and clearly impaired spatial learning. 4 SuMs is designated LH by Pan and McNaughton (2002) following Paxinos and Watson. 151 5.3. Exploratory and defensive behaviour Defensive behaviour has been categorised into three levels (Blanchard and Blanchard, 1988). Exploratory behaviour (when danger is uncertain or weak) is the first level of defense and so we have included it in the current section. The second level is that of freezing or avoidance when danger has been identified but is not immediate. The third level involves fight/flight or rage/panic when danger is immediate. The open field is a common test for exploration (the first level of defense). Neurotoxic lesions of the core of SuM increase exploratory ambulation but do not reduce the average frequency of theta (Pan and McNaughton, 2002). Electrolytic lesions of SuMp and SuMg also appear not to affect theta frequency during ambulation (Thinschmidt et al., 1995). Interestingly, injection of a benzodiazepine into SuM had no effect on theta frequency in the open field, but did reduce theta frequency when this was elicited by reticular stimulation (Pan and McNaughton, 1996). This confirms that a particular treatment can affect theta elicited by one stimulus while not affecting theta elicited by a second stimulus. Lesions of SuMs did not impair behaviour and theta in this task. Probably the simplest test for the next level of defense is fear conditioning (LeDoux, 1995). An interesting point about fear conditioning in relation to the hippocampus is that hippocampal lesions impair conditioning of fear to the context in which conditioning is carried out but do not impair conditioning to the discrete fear stimulus itself (Phillips and LeDoux, 1992, 1994). Neurotoxic lesions of the core of SuM also decrease contextual fear conditioning (i.e. decrease freezing and so increase movement) without affecting conditioning to the discrete stimulus (Pan and McNaughton, 2002). Lesions of the mammillary bodies, including SuM, also do not affect taste aversion conditioning (Sziklas and Petrides, 1993). A related test is passive avoidance (Gray and McNaughton, 1983). This has been tested with reversible inactivation of SuM by lidocaine (Shahidi et al., 2004). This has the disadvantage relative to neurotoxic lesions that effects can be due to either cells or fibres of passage but has the advantage that the time of inactivation can be varied. They found that retention (but not immediate acquisition) of passive avoidance was impaired by SuM inactivation either before or 5 min after acquisition. However, SuM inactivation at 90 or 360 min after acquisition or inactivation during the retention test did not have this effect. Importantly, this study (particularly with the effects obtained with injections 5 min after training) suggests that SuM is involved in the consolidation of the acquired information rather than initial learning or later retention. Consistent with this (but potentially a result of state dependency or spread to the mammillary bodies) injections of benzodiazepines into SuM appear to release punished (but not unpunished) responding in a conflict schedule (Kataoka et al., 1982) and lesion of the 152 W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 mammillary area including SuM increased approach and aggression towards an intruder in rats (Olivier et al., 1983). 5.4. Behavioural inhibition Passive avoidance is a test not only of defense but also of behavioural inhibition—a postulated fundamental function of the hippocampus (Gray, 1982; Gray and McNaughton, 2000). Behavioural inhibition can be tested without the use of shock in non-reward schedules such as FI and DRL (see Table 1). With FI, injections of benzodiazepine into SuMp produce increased responding and a concomitant decrease in theta frequency—and these effects are as large as the effects of i.p. injection of benzodiazepine (Woodnorth and McNaughton, 2002a,b). The benzodiazepine antagonist, flumazenil, injected into SuM also totally blocks the effect of the systemic drug on theta frequency (Woodnorth and McNaughton, 2002a,b). This suggests a particularly strong involvement of SuM with behavioural inhibition, in contrast to its weak involvement in spatial learning. Interestingly, neurotoxic lesions of either the core (SuMp + SuMg) or the shell (SuMs) increase responding and so impair inhibition. In contrast to defensive behaviour, however, and consistent with the effects of benzodiazepine injections in the same task, the change in behaviour produced by lesions of the SuM core is accompanied by a clear decrease in theta frequency of about 0.4 Hz (Pan and McNaughton, 2002). Lesions of SuMs by contrast did not reduce theta frequency. There was also a different response pattern between lesions of the core and the shell. The rats with core lesions showed more responding at the end rather than the beginning of the fixed interval, while the rats with shell lesions showed a high response rate over the whole interval. Neurotoxic lesions of the SuM core also produced increased responding in DRL. The results here are particularly interesting as theta could be recorded in relation to both rewarded and non-rewarded trials (in FI the vast majority of responses are non-rewarded). SuM lesions decreased theta frequency (by about 0.4 Hz) immediately prior to non-rewarded responses but not immediately prior to rewarded responses. This shows, within a single task, a dissociation of involvement and lack of involvement of SuM in the control of theta and, most importantly, it does so under conditions where the nature of the motor response is essentially the same. This suggests that SuM is involved in the control of theta under some psychological circumstances but not others. 5.5. SuM lesions compared to hippocampal lesions This review has focussed to an extent on the relation between SuM and the hippocampus. SuM is involved in the control of hippocampal theta activity and also, apparently via separate neurones, in hippocampal excitability and plasticity. However, both in terms of the different types of neurones in SuM and in terms of its diverse connections with structures other than the hippocampus we should expect only partial, and indeed quite small, overlap between supramammillary and hippocampal function. The observed overlap between the behavioural effects of supramammillary lesions and hippocampal lesions, however, is at present almost total (Table 2). This might not be surprising since the tasks have largely been chosen to test a supramammillary–hippocampal relation. They would not, therefore, be likely to demonstrate effects of supramammillary lesions that did not mimic hippocampal lesions. However, there are two important points to note here. First, with the exception of the radial arm maze, in all the cases so far tested if hippocampal lesions have an effect then supramammillary lesions or injections of chlordiazepoxide also have an effect. Supramammillary effects are of course smaller. The supramammillary lesions (or drug injections) produce subtotal dysfunction. Further, even a total loss of theta rhythm (and perhaps hippocampal non-theta modulation) should not produce the equivalent of total removal of the hippocampus. Nonetheless the qualitative pattern of positive results is surprisingly complete. The case where hippocampal lesions are without effect (simple fear conditioning) also shows complete concordance—a Table 2 The effects of SuM or hippocampal dysfunction on behaviour Task Open field Fear conditioning (CS) Fear conditioning (context) Passive avoidance FI (rate of response) DRL (rate of response) Spatial-conditional Water maze Measure Activity Activity Activity Activity Activity Activity Learning Learning SuM Hippocampus Core Shell *a ,a *a *d *a,f *a ,i ,a, +j ,a ,a ,a ? *a *a ,i ,a **b ,c **c **e **g **h ++i +++k Measure increased (*) or decreased (+) or unchanged (,). More arrows indicate larger effects. Question marks indicate marginal and/or inconsistent effects. All SuM effects (except passive avoidance) confirmed with fibresparing techniques (neurotoxic lesion or synaptically active agent injected into SuM). For a detailed review of the effects of hippocampal and septal lesions see Gray and McNaughton (1983). a Pan and McNaughton (2002). Note: SuMs is designated LH by Pan and McNaughton (2002) following Paxinos and Watson. b Rossi-Arnaud and Ammassari-Teule (1992), Earley et al. (1992), Stefanski et al. (1993) and Cannon et al. (1992). c Phillips and LeDoux (1994, 1995). d Shahidi et al. (2004). e Best and Orr (1973), Brunner and Rossi (1969), Kveim et al. (1964) and Miller et al. (1975). f Woodnorth and McNaughton (2002). g Manning and McDonough (1974) and Rabe and Haddad (1968). h Jarrard and Becker (1977), Acsádi et al. (1986), Woodruff et al. (1987) and Tonkiss et al. (1988). i Sziklas and Petrides (1998). j Pan and McNaughton (1997). k Morris et al. (1982) and Moser et al. (1995). W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 dissociation of simple fear conditioning from contextual fear conditioning by supramammillary lesions. Taken together, the studies summarised in Table 2 show that dysfunction of the core of SuM (the shell has not been specifically tested) in rats produces a pattern of changes in both simple motivated and emotional behaviours that is qualitatively similar to the pattern produced by hippocampal lesions. This said, it should be noted that effects on spatial learning in the water maze were barely detectable with benzodiazepine injection and essentially absent in rats with small neurotoxic lesions that were adequate to produce effects in other tasks. The Morris water maze is highly sensitive to hippocampal lesions (Morris et al., 1982; McDonald and Hong, 2000) and this suggests that SuM may be more involved in changes in emotional behaviour (as exemplified by behavioural inhibition according to Gray and McNaughton (2000)) that can be produced by hippocampal lesions than by changes in cognition. The apparent lack of effect of SuM (and mammillary) damage in the radial arm maze is consistent with this suggestion. On the other hand, it should be born in mind that, to produce relatively specific medial SuM dysfunction, the lesions used were far from complete in the anterior, posterior and lateral directions as will have been true also of the spread of injected drugs. Estimation of the extent of involvement of SuM in these behaviours would require total lesion of SuM with appropriate control lesions damaging the same areas outside SuM while sparing SuM itself as far as possible. It also seems likely that damage to both SuMg and SuMp would be required to maximize elimination of hippocampal modulation by SuM. 6. SuM, behavioural change and theta frequency The neurophysiological data make it clear that SuM is not the only nucleus involved in the control of theta. With reticular stimulation even large lesions of SuM produce only a modest reduction in theta frequency (McNaughton et al., 1995). The behavioural data reinforce this picture with, in the DRL task, a lack of change in theta frequency immediately before rewarded responses and a reduction in theta frequency immediately before non-rewarded responses. Given that lever pressing is likely to be stereotyped and given that theta frequency anticipates upcoming movement (Morris and Hagan, 1983) this strongly suggests that the control of theta frequency shifts between SuM and other areas depending on immediate task requirements. While the DRL task suggests highly labile control it does not allow us to dissect out the possible functional relationships between the control of theta by SuM and its involvement in behaviour. Here the results in the Morris water maze are of particular interest. SuM dysfunction had either a very small or no effect on theta frequency and other treatments (including reduction of 153 temperature with which SuM dysfunction appeared to interact) that reduced theta frequency also reduced spatial learning in proportion to the frequency reduction. (See below considerations of relative frequency reduction versus absolute frequency achieved.) Likewise, in FI, benzodiazepine injection into SuM produced an equivalent reduction in theta frequency to that of systemic injection and concurrently produced an equivalent change in behaviour. In all these cases, then, a sufficient reduction in theta frequency appeared to produce a change in behaviour. Conversely the minimal effects of SuM dysfunction on behaviour could, potentially, be attributed to its minimal effects on theta frequency. 6.1. Theta frequency change and behaviour: relative change or absolute value? Where there are relatively large changes in theta frequency we cannot tell whether it is the size of reduction in frequency that is important or a transfer of treated animals from above to below a critical functional threshold for frequency. Where changes are small, as with SuM dysfunction, it becomes possible that the same relative change might affect behaviour or not depending on the absolute frequency achieved in the control and treated group. Poor performance might be observed only in treated (or control) rats whose frequency was below a particular threshold value. Certainly, changing theta frequency seems to be the common link between the behavioural effects of quite different treatments. Decrease of body temperature or systemic benzodiazepine both change theta frequency and to similar extents. They also impair spatial working memory to similar extents. Givens and Olton (1990, 1994, 1995) and Givens (1995) also suggested spatial working memory is theta-dependent. They tested working memory in a continuous conditional discrimination task and reference memory in a sensory discrimination task after infutions of tetracaine, scopolamine, or muscimol into the medial septum or the nucleus basalis magnocellularis (NBM). MS infusion suppressed theta activity (amplitude) and impaired working memory but not reference memory. NBM infusion impaired reference memory but did not change theta. In a T-maze task, tetracaine, scopolamine, or muscimol into the medial septum decreased spatial working memory and theta, and so did low doses of ethanol. The pattern is more complex when we look at the smaller effects produced by SuM lesions. In the water maze, Pan and McNaughton (2002) found no effect on spatial reference memory and only slight signs of an effect on spatial working memory with lesions that reduced theta frequency from 7.5 to 7.1 Hz (0.4 Hz). By contrast, a significant (albeit small) effect on spatial working memory was obtained by Pan and McNaughton (1997) with benzodiazepine injections but: (a) this effect only appeared later in training when theta frequency was reduced (relative 154 W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 to control) from 6.0 to 5.5 Hz (0.5 Hz) and (b) was not seen earlier when theta frequency was reduced from 7.5 to 7.15 Hz (0.35 Hz). Pan (2000) reanalyzed the raw data of Pan and McNaughton (1997) to investigate the detailed relationship between frequency and spatial learning. He analyzed the relationship between theta frequency and performance efficiency over trial blocks 3–4, 5–6 and 7–8 in both the control (SuM saline injected) and treated (SuM benzodiazepine injected) rats. As can be seen in Fig. 9, the water maze performance tended to be poor when the theta frequency dropped below about 6.5 Hz in both control and treated rats. Water maze performance appeared good when theta frequency was above about 7 Hz. These water maze data suggest that there may be a threshold in the region of 6.5 Hz below which frequency must drop to produce substantial impairments. Consistent with this, systemic benzodiazepine and ‘‘long cooling’’ exposure decrease the frequency to 6.5 Hz at the beginning of training and impair learning clearly, but ‘‘short cooling’’ exposure decreases theta frequency to 6.8 Hz and has no significant effect on the learning (Pan and McNaughton, 1997). With increasing dose of ethanol, frequency decreased and at 1.0 g/kg, when a clear impairment of spatial working memory was obtained, theta frequency dropped to about 6.38 Hz (Givens, 1995). With operant schedules the same kind of pattern emerges. Pan and McNaughton (2002) obtained reductions from 7.3 to about 6.75 Hz (0.55 Hz) with clear but relatively modest changes in behaviour. Woodnorth and McNaughton (2002a,b) obtained reductions from 7.0 to 6.3 Hz (0.7 Hz) and obtained a substantial change in behaviour (indeed one identical in size to systemic injection of the benzodiazepine). There is a tendency for larger reductions in frequency to have been produced against the background of a lower control frequency and so these data cannot easily separate the effect of the size of reduction from the absolute value of theta frequency obtained. However, the individual data shown in Fig. 9 suggest that it is the reduction of frequency of theta below a critical threshold value that is functionally important rather than the relative difference between the theta frequencies of treated and control rats. That said, in both cases, it is clear that some form of threshold is operating. Behaviour is not changed until a sufficiently substantial effect is obtained on theta. The same may be true of increases in frequency above the norm. This suggests that future experiments may need both to monitor theta frequency and to attempt to produce dysfunction that is both large and specific. Fig. 9. Relationship between theta frequency and performance in the water maze. (A) In general over the course of training (trials 3–4, 5–6, 7–8, data across the pair of trials were averaged for each individual rat) there is an inverse relationship between theta frequency and distance traveled to reach the hidden platform. Thus a higher frequency is related to better performance. This relationship is essentially similar for both rats receiving injections of the benzodiazepine chlordiazepoxide (CDP) in SuM and saline (SAL) injected controls (compare fitted curves). There is some suggestion that this effect is due to a deterioration in performance when the frequency drops below a threshold value of about 6.5 Hz (dashed vertical line). This suggestion is supported by the data in B. (B) Manipulations of SuM produced a change in theta frequency relative to their controls that was as large as that of cooling water relative to its control. Yet the latter had a much greater effect on performance. Systemic administration of CDP produced a greater change in frequency relative to its control than did cooling water, but these two manipulations reduced frequency to about the same absolute value (below 6.5 Hz) while both their controls were above 6.5 Hz, and they produced similar changes in performance relative to their controls. Data re-analyzed from Pan and McNaughton (1997, 2002). W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 6.2. Changes in behaviour with no absolute change in theta frequency In the water maze and operant schedules theta and behaviour both changed. However, SuM lesion did not change theta frequency during either of the defensive tasks in which it was measured, despite its ability to change behaviour. One possible explanation of this is that the increase in movement produced by the lesions produced an increase in theta. This increase in movement then counteracted a concurrent lesion-induced decrease in theta frequency. Certainly the normal relation between movement and frequency must have been disturbed or an increase in theta frequency would otherwise have been observed. However, it is difficult to see this providing a total explanation of a lack of frequency reduction. The data suggest, then, that changes in defensive behaviour produced by SuM dysfunction are not totally mediated by changes in theta. There are two ways in which an uncoupling of defensive behaviour and theta is consistent with other data in this paper. First, there is modulation by SuMg of the hippocampus that is independent of theta. We would expect, therefore, at least some SuM-induced changes in behaviour mediated by the hippocampus to be independent of theta. Secondly, SuM has extensive non-hippocampal connections, including direct connections with the defense system. Indeed, in this context, its connections with the hippocampus could be viewed as just another part of its widespread connection with the limbic system in general. Here any parallels with the behavioural effects of hippocampal lesions could be the result of output from both SuM and hippocampus affecting common targets. In relation to defense, indeed, even the functional links between SuM and the hippocampus are likely to involve not only ascending pathways but also descending. There are connections from the septo-hippocampal system, via LS to SuM and thence to CG. This would allow SuM to mediate the effects of hippocampal output on defensive behaviour without interacting with the outputs that alter spatial learning. This suggestion is consistent with the fact that water maze learning involves quite different systems from defensive behaviour (Roozendaal and McGaugh, 1997; Roozendaal et al., 1996, 1998). SuM also connects to the amygdala and the amygdala–CG path is regarded as a central part of an emotional system (LeDoux, 1995; Fendt and Fanselow, 1999). We would, therefore, expect SuM to alter defensive behaviours in addition to those affected by hippocampal lesions. Here, the results with fear conditioning are somewhat surprising. Lesions of the septal (dorsal) portion of the hippocampus affect contextual fear conditioning only (Phillips and LeDoux, 1994, 1995). This dorsal hippocampal control appears to be mediated by interaction with the amygdala (LeDoux, 1995). The amygdala, by contrast, controls both contextual and CS conditioning of fear 155 (LeDoux, 1993a,b). Yet, despite its direct connections to the amygdala, SuM lesions act more like dorsal hippocampal than amygdala lesions. There are indications that the temporal (ventral) portion of the hippocampus is involved in both simple and contextual fear conditioning, like the amygdala, and is relatively uninvolved in spatial learning (Richmond et al., 1999). We will argue shortly that SuM receives information more from the septal portion than the temporal portion of the hippocampus. This would account for its involvement in contextual as opposed to simple fear conditioning. But it leaves unclear why it should be so little involved in spatial learning. These data suggest that future work may need to explore different defensive behaviours to a greater extent and/or investigate the effects of more complete SuM lesions. Equally, given the heterogeneity of the amygdala, which is so great that it cannot be viewed as a distinct structural entity (Swanson and Petrovich, 1998), the targets of SuM and hippocampal connections in this region could prove to be common and to be distinct from the portions of the amygdala that mediate simple fear conditioning. 7. SuM—an interface between cognition and emotion? All the above suggests that SuM may be an important cross-road linking higher cognitive and lower emotional structures—so mediating cognitive–emotional interactions. Here, rostral forebrain–SuM–CG pathways may be of particular importance. SuM connects bidirectionally with a range of structures including LS, ventromedial prefrontal areas and the cingulate (Fig. 5). The LS plays a clear role in defensive behaviour (Risold and Swanson, 1997). Lesions of the ventromedial prefrontal cortex (prelimbic/infralimbic cortex) impair anxiety-related behaviours, passive avoidance, and delayed response tasks often producing perseveration but, like lesions of SuM, they do not affect behaviour in the Morris water maze (Heidbreder and Groenewegen, 2003). Anterior cingulate lesions can increase conditioned fear responses, but also can ‘‘reduce the aversiveness or perceived unpleasantness of nociceptive stimuli’’ and impair some avoidance tasks as well as ‘‘produce dysfunctions in the motor planning resulting from nociceptor stimulation’’ (Heidbreder and Groenewegen, 2003). Like ventral prefrontal cortex and SuM, anterior cingulate does not appear to be involved in spatial learning (Heidbreder and Groenewegen, 2003). SuM bidirectionally connects with the CG. CG is a critical output structure for emotional behaviours. It mediates the behavioural output of fear conditioning (LeDoux et al., 1988; Wilson and Kapp, 1994; Fendt and Fanselow, 1999), or fear to a natural predator (Canteras and Goto, 1999), as well as anxiety-related behaviour in the open field (McCarthy et al., 1995) and elevated plus-maze (Schmitt et al., 1990, 2001; Silveira et al., 1993; Souza et al., 1998). Similarly, SuM has bidirectional connections with the 156 W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 raphe nuclei and a variety of hypothalamic structures all of which are involved in the control of motivation and emotion. Notwithstanding these bidirectional connections of SuM, this review has presented a picture of SuM as tightly linked to the hippocampus and related caudal limbic structures. In part this is driven by the importance (and even now the enigmatic nature) of the hippocampus coupled with the role of SuM in hippocampal modulation and the control of hippocampal theta activity. In greater part it is driven by the fact that much of the research on SuM has taken a hippocampal focus. However, one could also justify this focus if we take the view that the bidirectional connections of SuM reflect its involvement in a distributed system that assesses the emotional and motivational value of stimuli while its unidirectional connections represent outputs that control cognitive processing. (There are of course, descending feedback loops from caudal limbic structures to SuM but these appear both less direct and less powerful than the ascending inputs to them from SuM.) In this final section, therefore, we will discuss the functions of SuM in relation to the hippocampus before taking a more general point of view. 7.1. SuM, behaviour and hippocampal function For the purposes of this section we will treat the entorhinal cortex and hippocampus proper as parts of an extended hippocampal formation (Gray and McNaughton, 2000). Both show theta activity controlled by MS and both receive direct input from more lateral portions of SuM and send feedback to SuM that is relayed in the septum (Fig. 3). SuM dysfunction produces hyperactivity in defensive tasks, including passive avoidance, and non-reward schedules (increased bar pressing). This is similar to, but much weaker than, the effect of hippocampal dysfunction in those tasks. A critical point is that the hippocampal parallel extends to a specific effect on contextual fear conditioning with no effect on simple fear conditioning. In this respect, SuM dysfunction is unlike amygdalar dysfunction. However, SuM dysfunction has relatively little effect on spatial learning in the water maze task. This dissociation is made most clear by comparing injection of benzodiazepines into SuM with systemic injections. In the water maze, systemic benzodiazepine has substantial effects on both theta frequency and behaviour; SuM injection has little effect on either (Pan and McNaughton, 1997). By contrast, in FI both types of injection have the same substantial effects on both theta frequency and behaviour (Woodnorth and McNaughton, 2002a,b). These data suggest that SuM plays a more important role in relation to the involvement of the hippocampus in behavioural inhibition (emotion) than spatial learning (cognition). This is consistent with the c-fos studies discussed earlier that implicated SuM in emotional behaviours. This raises a question: how does SuM dysfunction impair emotional behaviours in ways that are similar to those produced by hippocampal lesion if the same level of SuM dysfunction does not have the same magnitude of effect on the paradigm test of hippocampal function— the water maze. This question can be answered in two ways. First, the relative lack of effect of SuM dysfunction in the water maze is paralleled by a lack of effect on theta frequency. The control of theta in the water maze (and its benzodiazepine sensitivity) has partially transferred to another structure. From this we can deduce (a) that larger SuM dysfunction would show somewhat larger effects, but (b) given the data with chlordiazepoxide, SuM involvement in the water maze will never be as complete as in, for example, FI. Conversely, in defensive situations and in schedules requiring behavioural inhibition, SuM is more substantially in control of theta and so can demonstrate a greater functional contribution to behaviour. Second, we need to consider the outputs of the hippocampus. There is no evidence that its control of spatial learning or spatial working memory is mediated by defensive areas such as the amygdala. By contrast, its control of defense would be expected to be mediated by outputs to the amygdala and CG. SuM output is sent to the same targets and so could produce the same effects as hippocampal lesions (on defense)—but without requiring any mediation by the hippocampus. This explanation appears particularly likely for defensive tasks—as these demonstrate substantially greater effects of SuM function on behaviour than on theta relative to systemic benzodiazepine injections. Mediation of the effects of SuM dysfunction by the amygdala appears unlikely for FI—as this task is not affected by lesions of the amygdala (Gray and McNaughton, 2000). Further, FI demonstrates essentially the same effects of SuM dysfunction on behaviour as theta relative to systemic benzodiazepine injection. Thus SuM is likely to have two parallel effects on tasks involving behavioural inhibition—an indirect effect on the amygdala, CG and other structures mediated by changes in hippocampal theta (which would themselves be larger than the changes in theta seen in the water maze) and direct effects on the amygdala and CG mediated by its outputs to these structures (which would be irrelevant for spatial learning). Thus SuM would be involved in both ascending and descending control of these behaviours. We would also expect future research to demonstrate additional parallels between SuM and hippocampal function. The treatments we have considered have usually targeted the core of SuM and, in addition, have often used benzodiazepine injections in attempts to restrict effects to the aspects of SuM that control theta. Further studies should selectively target SuMp, SuMg and SuMs and aim to use specific neurotoxic or pharmacological treatments aimed at the cells that can modulate hippocampal responses independently of theta. So far we have no specific evidence W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 that these SuMg cells have been affected significantly in any behavioural task. 7.2. SuM, the amygdala and thalamus Like the hippocampus and entorhinal cortex, the amygdala and thalamus primarily receive direct input from SuM with any feedback to SuM being relayed in other structures. To some extent all these targets of SuM afferents can be seen as dealing with more cognitively than emotionally loaded information. For example, as noted already, the connection of SuM with the amygdala (as the connection of the hippocampus with the amygdala) may modulate contextual fear conditioning but does not modulate simple fear conditioning. This said, we should remind ourselves (Section 7.1) that SuM appears not to be greatly involved in more cognitively loaded tasks. SuM, then, can be seen as dealing with classes of information that are to some extent common to the hippocampus and amygdala—the more emotionally loaded aspects of hippocampal function and the more cognitively loaded aspects of amygdalar function. The role of the thalamus is unclear in this context. However, it too can be seen as involving cognitive– emotional compounds if we can presume that SuM is making connections in the thalamus with components of the Papez circuit and so modulating information being passed from the mammillary bodies to the thalamus and so affecting interactions of the latter with neocortex (Parmeggiani et al., 1971, 1974). The direct output from SuM to the mammillary bodies (Gonzalo-Ruiz et al., 1992a,b) may be involved in similar control. We have argued previously that a critical function of theta activity may be to pace the passage of information round the Papez circuit and related hippocampal–neocortical loops (Gray and McNaughton, 2000) that are likely to circulate information at frequencies in the theta range (Miller, 1989, 1991). One particularly interesting suggestion relates to the mediodorsal thalamus. ‘‘We also found multi-unit activity coherent with hippocampal theta EEG in the medial dorsal nucleus of the thalamus. The medial dorsal nucleus does not receive input from the mammillary bodies so is unlikely to be part of the same re-entrant circuit as the anteroventral, anterodorsal and anteromedial nuclei. However, the medial dorsal thalamus is in receipt of afferent input from the SuM. . . . Medial dorsal thalamus–perirhinal and hippocampal–anterior thalamic systems may well be capable of independent theta generation and . . . independently coordinate task-related activity between nodes in their own network [dealing with] item recognition and episodic processes. . . . Appropriate coherent theta activity across particular nodes would facilitate transfer of information between systems’’ (Kirk and Mackay, 2003). It could be added that although Kirk and McKay locate the anteromedial thalamic nucleus within the cluster of thalamic nuclei supposedly receiving theta rhythmic information 157 from the hippocampus, via the mammillary bodies, it also (Fig. 5) receives direct input from SuM and so could be particularly involved in interactions between the two postulated memory systems. Also remaining to be fully categorised, but receiving direct unidirectional input from SuM are the central medial nucleus and nucleus reuniens. Under urethane, at least, the central medial nucleus (which like SuM and nucleus reuniens receives tonic, theta-related, input from the posterior hypothalamus) shows only tonic theta-related activity and so would appear to precede SuM in the generation of theta—likewise high-frequency stimulation of nucleus reuniens elicits rather than blocks theta (Bland et al., 1995; Kirk, 1998). 7.3. SuM and forebrain control of emotion The forebrain structures we have considered so far (Sections 7.1 and 7.2) return information to SuM only via relays and can either be thought of as primarily cognitively oriented (entorhinal cortex, hippocampus, thalamus) or interacting with SuM only to execute limited emotional functions (amygdala). Nonetheless, we argued that their involvement with SuM was linked to some degree of emotional content. We now turn to structures that are reciprocally connected to SuM, that are more fundamentally involved in emotion and that, interestingly, often receive output from the structures we have already considered. They are not only reciprocally connected with SuM, therefore, but are also often components of feedback loops to SuM. Potentially the most revealing of these structures is the LS. This is thought to be an important structure mediating defensive behaviour and receives topographically organised input from the hippocampal formation that it then relays, topographically, to the hypothalamus (Risold and Swanson, 1996). From this perspective, the various LS–SuM connections can be seen as parts of a parallel system that topographically maps specific parts of the hippocampal formation into specific parts of the hypothalamus. This system is likely to be involved in the hippocampal control of emotion, and perhaps more specifically goal-directed behaviour, more than cognition (Risold and Swanson, 1996). Similarly, the amygdala (like the hippocampus) receives input from SuM and projects to the medial hypothalamus and CG. The main cortical areas reciprocally connected to SuM are the infralimbic cortex, the dorsal peduncular cortex and the cingulate cortex. As we noted above these all have some claim to involvement in defensive behaviour (as well as other motivated behaviours) and all also have reciprocal connections to the amygdala. ‘‘Although the medial prefrontal cortex is clearly involved in a variety of cognitive and emotional processes, its dorsal and ventral subregions seem to be involved in different aspects of the information processes. Thus, the dorsal part of the medial 158 W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 prefrontal cortex (dorsal anterior cingulate and dorsal prelimbic areas) is mainly involved in the temporal patterning of behavioural sequences. In contrast, the ventral part of the medial prefrontal cortex (ventral prelimbic area, as well as infralimbic and medial orbital cortices) appears to be critical for the flexible shifting to new strategies or rules in spatial and visual-cued discrimination tasks and, perhaps even more importantly, for the integration of internal physiological states with salient environmental cues to guide behaviour in situations of perceived threat or exposure to aversive stimuli. . . . Prelimbic cortex [may be] responsible for voluntary responses (goal-directed initial responding), whereas the infralimbic cortex would mediate the progressive and incremental ability of overtraining to lead to behavioural autonomy and develop habits that are no longer voluntary or goal-directed’’ (Heidbreder and Groenewegen, 2003). The medial prefrontal cortex is topographically mapped into the MS/DBB (Fig. 10). ‘‘More ventral regions, including the infralimbic and ventral prelimbic areas, project more densely to the septum and medial areas of the basal forebrain, while the dorsal parts of the prelimbic area and the anterior cingulate area project more laterally to reach the horizontal limb of the diagonal band of Broca’’ (op. cit., p. 563). The medial septum and diagonal band, then project topographically to ventral (temporal) and dorsal (septal) portions of the hippocampus, respectively. The ventral and dorsal aspects of the hippocampus then project topographically via the LS (as noted above) to rostral and caudal hypothalamus, respectively. The mapping achieved by this putative polysynaptic pathway appears to be the same (infralimbic cortex to rostral hypothalamus, anterior cingulate to caudal hypothalamus) as the principle direct projections from these areas. Further, ‘‘the different regions of the medial prefrontal cortex . . . [also] project in a topographical way to functionally distinct parts of the [CG and so may] engage in parallel, functionally distinct ‘emotional motor’ circuits. These ‘emotional motor’ circuits [would] underlie different aspects of integrated behavioural and autonomic/endocrine responses, such as active and passive coping with stress’’ [op. cit., p. 569]. Thus the rostral limbic cortex and lateral septum not only share reciprocal connections with SuM but also appear to be part of an extensive topographically homogenous system (Fig. 10). They also all receive direct input from but do not project directly to the hippocampal formation. Taking all of the above areas together with the raphe nuclei and CG, we can conclude that SuM has direct reciprocal connections Fig. 10. Some selected structures and connections focussing on the role of SuM. SuM connects bidirectionally with rostral limbic cortex including the infralimbic (IL) and anterior cingulate (AC) cortex. As described in the text this cortex is topographically mapped into the septum and topographic mapping continues via the hippocampus, lateral septum and hypothalamus, as shown. (Note that dorsomedial septum is shown below the ventrolateral septum to simplify the topographic mapping.) The mapping is illustrated for only one dimension in each structure but is, in fact, two-dimensional (with respect to flat sheet representations of each level of the system). We postulate that the most extreme strands of this topographically organised system are specialised for more cognitive (AC, septal pole of hippocampus) and more emotional (IL, temporal pole of the hippocampus) processing—but with each representing goals and thus encoding conjunctions of cognitive and emotional features. This would be consistent with previous independent suggestions about the partitioning of function in the cortical structures (Heidbreder and Groenewegen, 2003) and the hippocampus (Richmond et al., 1999). It would also be consistent with, for example, the greater input from the amygdala to temporal portions of the hippocampus. In this descending scheme, SuM is just one of an array of hypothalamic structures receiving topographically mapped input, presumed to be involved in the control of goal-related behaviour (Risold and Swanson, 1996). The direct connections of the cortex to the hypothalamus appear to show the same mapping as the relayed connections. It is likely that the topographic mapping of the various parts of the system extends to their connections with the central gray (CG). In contrast to the descending connections the ascending connections of SuM are quite diffuse and passage of information around these recurrent circuits could, we postulate, control cognitive–emotional interactions. W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 with structures, including rostral components of the limbic system, that uniformly have a close involvement in the control of emotion. SuM also has unidirectional projections that connect it directly to more caudal aspects of the limbic system but these can then provide feedback relayed through the structures with which SuM has reciprocal connections. 7.4. SuM—a controller of cognitive–emotional interaction? Given the tentative functional circuitry proposed in Fig. 10, we can now approach a suggestion as to the role of SuM. The entorhinal cortex, hippocampus, thalamus (and possibly the SuM-related aspects of the amygdala) are likely to be involved in the processing of more cognitive components of emotional reactions. They all receive input from SuM and, directly or indirectly, can send output to alter the functioning of structures more intimately involved in the generation of emotional outputs. It seems likely, then, that a primary function of SuM is to modulate cognitive processing involved in emotional reactions. In this context, the control of theta activity by SuM is likely to be just one of a range of ascending influences it can exert on caudal limbic structures. An involvement of these circuits in stress is suggested both by the c-fos data in SuM and the fact that the hippocampus is a major site at which there are corticosterone receptors and it is involved in the control of the pituitary–adrenal axis (McEwen, 1994, 1999; McEwen et al., 1969). In turn, these primary targets of SuM output can send relayed feedback to SuM. If theta activity is a guide here, this relayed feedback is likely to be, in and of itself, fairly minor. We have evidence for hippocampal control of the number of action potentials per theta burst in SuM but this is related to only minor alteration in theta frequency itself (Kirk and McNaughton, 1991) and such alteration as can be demonstrated is an increase not a decrease in theta (Kirk et al., 1996)—suggesting simple negative feedback. Further, while the input of SuM to limbic cortical areas appears quite diffuse, their output to SuM appears to be just one part of a tightly topographically organized output to the hypothalamus as a whole. Thus, only parts of these structures project to SuM and the areas that do not project to SuM project, topographically, to other areas of the hypothalamus. If other parts of the hypothalamus contain additional controllers of theta (and additional systems that modulate plasticity) then we can imagine a system where rostral limbic areas, caudal limbic areas and the hypothalamus are all similarly interconnected with ascending connections being diffuse (one to all) and descending ones being tightly topographic (one to one). While there are some structures that appear to provide unidirectional input to SuM, it seems likely that the bulk of its significant input allows it to monitor activity in structures that control emotion (as with CG) or that evaluate significant emotional stimuli (infralimbic cortex, cingulate cortex). Again, we can perhaps use theta activity as a guide. The control of theta by SuM (as opposed to the control of theta by some other structure or structures) is evident only with more 159 emotionally loaded tasks (and even then is obvious only when movement is not increased). This would be simply achieved if the inputs to SuM (but less so inputs to other theta-controlling structures) are primarily from areas that control emotion. The higher is the intensity of such inputs, the higher is the frequency of theta. By analogy, then, non-theta components of SuM may also receive input from areas involved in the detection of, or response, to emotionally significant events and produce output in proportion to the level of input. The reciprocal connections from SuM to these emotionprocessing areas could fulfill either of the two functions. They could involve an initial crude attempt to modulate these structures, or an attempt to prime them, in anticipation of the outputs from the caudal limbic group of structures. Alternatively it could involve a fast feedback control of the activity of SuM that altered its output to the caudal limbic group of structures depending on the relative balance between activities in the structures of the reciprocal network. However, there is an extra dimension to the asymmetry of the ascending and descending connections to be considered. In the parallel system suggested by Heidbreder and Groenewegen (see previous section, solid lines in Fig. 10) only one strand nominally projects to SuM with the other strands targeting other areas of the hypothalamus. Further, from a hippocampal perspective, this feedback is associated with the septal rather than the temporal pole. The septal pole receives relatively little input from the amygdala compared to the temporal pole (Amaral et al., 1992). Consistent with this the septal pole appears to be more selectively involved in spatial processing and the temporal pole more selectively involved in anxiety (Richmond et al., 1999). Yet SuM, itself, seems to have a relatively lesser involvement in spatial processing than anxiety. Thus the ascending connections from SuM may be more concerned with adding an emotional influence to cognitive processing while its descending connections may be more concerned with adding a cognitive influence to emotional processing. Both more detailed anatomy and more, less hippocampally oriented, physiology and behavioural analysis will be required before we can fully understand the complex web of neural interactions within which the supramammillary area must be embedded. References Acsádi, G., Buzsáki, G., Keszthelyi, T., Királyfalvi, L., Gage, F.H., 1986. Effects of confinement, previous experience and hippocampal damage on the DRL schedule. Behav. Brain Res. 20, 241–248. Amaral, D.G., Cowan, W.M., 1980. Subcortical afferents to the hippocampal formation in the monkey. J. Comp. Neurol. 189, 573–591. Amaral, D.G., Price, J.L., Pitkänen, A., Carmichael, S.T., 1992. Anatomical organization of the primate amygdaloid complex. In: Aggleton, J.P. (Ed.), The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction. Wiley, pp. 1–66. Ammassari-Teule, M., Maho, C., Sara, S.J., 1991. Clonidine reverses spatial learning deficits and reinstates theta frequencies in rats with partial fornix section. Behav. Brain Res. 45, 1–8. 160 W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 Anchel, H., Lindsley, D.B., 1972. Differentiation of two reticulo-hypothalamic systems regulating hippocampal activity. Electroencephalogr. Clin. Neurophysiol. 32, 209–226. Apostol, G., Creutzfeldt, O.D., 1974. Crosscorrelation between the activity of septal units and hippocampal EEG during arousal. Brain Res. 67, 65–75. Assaf, S.Y., 1978. Electrical activity in the hippocampal formation of the rat: role of ascending monoamine systems. Unpublished Ph.D. Thesis. University of British Columbia. Assaf, S.Y., Mason, S.T., Miller, J.J., 1979. Noradrenergic modulation of neuronal transmission between the entorhinal cortex and the dentate gyrus of the rat. J. Physiol. 292, 65P–65P. Assaf, S.Y., Miller, J.J., 1978. Neuronal transmission in the dentate gyrus: role of inhibitory mechanisms. Brain Res. 151, 587–592. Ball, G.G., Gray, J.A., 1971. Septal self-stimulation and hippocampal activity. Physiol. Behav. 6, 547–549. Beck, C.H.M., Fibiger, H.C., 1995. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J. Neurosci. 15, 709–720. Berger, B., Esclapez, M., Alvarez, C., Meyer, G., Catala, M., 2001. Human and monkey fetal brain development of the supramammillary–hippocampal projections: a system involved in the regulation of theta activity. J. Comp. Neurol. 429, 515–529. Best, P.J., Orr, J., 1973. Effects of hippocampal lesions on passive avoidance and taste aversion conditioning. Physiol. Behav. 10, 193–196. Bhatnagar, S., Costall, B., Smythe, J.W., 1997. Hippocampal cholinergic blockade enhances hypothalamic–pituitary–adrenal responses to stress. Brain Res. 766, 244–248. Blanchard, D.C., Blanchard, R.J., 1988. Ethoexperimental approaches to the biology of emotion. Annu. Rev. Psychol. 39, 43–68. Bland, B.H., 1986. The physiology and pharmacology of hippocampal formation theta rhythms. Prog. Neurobiol. 26, 1–54. Bland, B.H., Colom, L.V., 1993. Extrinsic and intrinsic properties underlying oscillation and synchrony in limbic cortex. Prog. Neurobiol. 41, 157–208. Bland, B.H., Konopacki, J., Kirk, I.J., Oddie, S.D., Dickson, C.T., 1995. Discharge patterns of hippocampal theta-related cells in the caudal diencephalon of the urethan-anesthetized rat. J. Neurophysiol. 74, 322–333. Bland, B.H., Oddie, S.D., 1998. Anatomical, electrophysiological and pharmacological studies of ascending brainstem hippocampal synchronizing pathways. Neurosci. Biobehav. Rev. 22, 259–273. Bland, B.H., Oddie, S.D., 2002. Theta band oscillation and synchrony in the hippocampal formation and associated structures: the case for its role in sensorimotor integration. Behav. Brain Res. 127, 119–136. Bland, B.H., Oddie, S.D., Colom, L.V., Vertes, R.P., 1994. Extrinsic modulation of medial septal cell discharges by the ascending brainstem hippocampal synchronizing pathway. Hippocampus 4, 649–660. Bland, B.H., Trepel, C., Oddie, S.D., Kirk, I.J., 1996. Intraseptal microinfusion of muscimol: effects on hippocampal formation theta field activity and phasic theta-ON cell discharges. Exp. Neurol. 138, 286– 297. Borhegyi, Z., Freund, T.F., 1998. Dual projection from the medial septum to the supramammillary nucleus in the rat. Brain Res. Bull. 46, 453–459. Borhegyi, Z., Leranth, C., 1997. Distinct substance P- and calretinincontaining projections from the supramammillary area to the hippocampus in rats: a species difference between rats and monkeys. Exp. Brain Res. 115, 369–374. Borhegyi, Z., Maglóczky, Z., Acsády, L., Freund, T.F., 1998. The supramammilary nucleus innervates cholinergic and gabaergic neurons in the medial septum-diagonal band of Broca complex. Neuroscience 82, 1053–1065. Born, J., Fehm, H.L., 1998. Hypothalamus–pituitary–adrenal activity during human sleep: a coordinating role for the limbic hippocampal system. Exp. Clin. Endocrinol. Diab. 106, 153–163. Borst, J.G.G., Leung, L.-W.S., MacFabe, D.F., 1987. Electrical activity of the cingulate cortex. II. Cholinergic modulation. Brain Res. 407, 81–93. Bose, A., Recce, M., 2001. Phase precession and phase-locking of hippocampal pyramidal cells. Hippocampus 11, 204–215. Brake, W.G., Sullivan, R.M., Flores, G., Srivastava, L.K., Gratton, A., 1999. Neonatal ventral hippocampal lesions attenuate the nucleus accumbens dopamine response to stress: an electrochemical study in the adult rat. Brain Res. 831, 25–32. Brazhnik, E.S., Fox, S.E., 1997. Intracellular recordings from medial septal neurons during hippocampal theta rhythm. Exp. Brain Res. 114, 442–453. Brucke, F., Petsche, H., Pillat, B., Deisenhammer, E., 1959. Die Beeinflussung der ‘Hippocampus-arousal-Reaktion’ beim Kaninchen durch elektrische Reizung im Septum. Pflugers Arch. Ges. Physiol. 269, 319–338. Brunner, R.L., Rossi, R.R., 1969. Hippocampal disruption and passive avoidance behavior. Psychon. Sci. 15, 228–229. Cannon, R.L., Paul, D.J., Baisden, R.H., Woodruff, M.L., 1992. Alterations in self-grooming sequences in the rat as a consequence of hippocampal damage. Psychobiology 20, 205–218. Canteras, N.S., Goto, M., 1999. Fos-like immunoreactivity in the periaqueductal gray of rats exposed to a natural predator. Neuroreport 10, 413–418. Carnes, K.M., Fuller, T.A., Price, J.L., 1990. Sources of presumptive glutamatergic/aspartatergic afferents to the magnocellular basal forebrain in the rat. J. Comp. Neurol. 302, 824–852. Carre, G., Harley, C., 1991. Population spike facilitation in the dentate gyrus following glutamate to the lateral supramammillary nucleus. Brain Res. 568, 307–310. Chiba, T., Murata, Y., 1985. Afferent and efferent connections of the medial preoptic area in the rat: a WGA-HRP study. Brain Res. Bull. 14, 261–272. Chrobak, J.J., Hanin, I., Walsh, T.J., 1987. AF64A (ethylcholine aziridinium ion), a cholinergic neurotoxin, selectively impairs working memory in a multiple component T-maze task. Brain Res. 414, 15–21. Chrobak, J.J., Lörincz, A., Buzsáki, G., 2000. Physiological patterns in the hippocampo-entorhinal cortex system. Hippocampus 10, 457–465. Cohen, N.J., Eichenbaum, H., 1993. Memory, Amnesia, and the Hippocampal Memory System. The MIT Press, Cambridge, MA. Conrad, L.C., Pfaff, D.W., 1976. Efferents from medial basal forebrain and hypothalamus in the rat. II. An autoradiographic study of the anterior hypothalamus. J. Comp. Neurol. 169, 221–261. Contestabile, A., Flumerfelt, B.A., 1981. Afferent connections of the interpeduncular nucleus and the topographic organization of the habenulo-interpeduncular pathway: an HRP study in the rat. J. Comp. Neurol. 196, 253–270. Cullinan, W.E., Helmreich, D.L., Watson, S.J., 1996. Fos expression in forebrain afferents to the hypothalamic paraventricular nucleus following swim stress. J. Comp. Neurol. 368, 88–99. Deacon, T.W., Eichenbaum, H., Rosenberg, P., Eckmann, K.W., 1983. Afferent connections of the perirhinal cortex in the rat. J. Comp. Neurol. 220, 168–190. Destrade, C., 1982. Two types of diencephalically driven RSA (theta) as a means of studying memory formation in mice. Brain Res. 234, 486–493. Destrade, C., Ott, T., 1982. Is a retrosplenial (cingulate) pathway involved in the mediation of high frequency hippocampal rhythmical slow activity (theta)? Brain Res. 252, 29–37. Dickson, C.T., Kirk, I.J., Oddie, S.D., Bland, B.H., 1995. Classification of theta-related cells in the entorhinal cortex: cell discharges are controlled by the ascending brainstem synchronizing pathway in parallel with hippocampal theta-related cells. Hippocampus 5, 306–319. Dutar, P., Rascol, O., Lamour, Y., 1989. Rhythmical bursting activity and GABAergic mechanisms in the medial septum of normal and pertussis toxin-pretreated rats. Exp. Brain Res. 77, 374–380. Earley, B., Burke, M., Leonard, B.E., 1992. Behavioural, biochemical and histological effects of trimethyltin (TMT) induced brain damage. Neurochem. Int. 21, 351–366. W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 Fendt, M., Fanselow, M.S., 1999. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci. Biobehav. Rev. 23, 743– 760. Ferrini, M., Piroli, G., Frontera, M., Falbo, A., Lima, A., De Nicola, A.F., 1999. Estrogens normalize the hypothalamic–pituitary–adrenal axis response to stress and increase glucocorticoid receptor immunoreactivity in hippocampus of aging male rats. Neuroendocrinology 69, 129–137. Freund, T.F., Antal, M., 1988. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature 336, 170–173. Frotscher, M., Leranth, C., 1985. Cholinergic innervation of the rat hippocampus as revealed by choline-acetytransferase immunocytochemistry: a combined light and electron-microscope study. J. Comp. Neurol. 239, 237–246. Fujita, Y., Sato, T., 1964. Intracellular records from hippocampal pyramidal cells in rabbit during theta rhythm activity. J. Neurophysiol. 27, 1011– 1025. Gall, C., Selawski, L., 1984. Supramammillary afferents to guinea pig hippocampus contain substance P-like immunoreactivity. Neurosci. Lett. 51, 171–176. Gestreau, C., Le Guen, S., Besson, J.M., 2000. Is there tonic activity in the endogenous opioid systems? A c-Fos study in the rat central nervous system after intravenous injection of naloxone or naloxone-methiodide. J. Comp. Neurol. 427, 285–301. Givens, B., 1995. Low doses of ethanol impair spatial working memory and reduce hippocampal theta activity. Alcoholism (NY) 19, 763–767. Givens, B., 1996. Stimulus-evoked resetting of the dentate theta rhythm: relation to working memory. Neuroreport 8, 159–163. Givens, B., Olton, D.S., 1994. Local modulation of basal forebrain: effects on working and reference memory. J. Neurosci. 14, 3578–3587. Givens, B., Olton, D.S., 1995. Bidirectional modulation of scopolamineinduced working memory impairments by muscarinic activation of the medial septal area. Neurobiol. Learn. Mem. 63, 269–276. Givens, B.S., Olton, D.S., 1990. Cholinergic and GABAergic modulation of medial septal area: effect on working memory. Behav. Neurosci. 104, 849–855. Gogolák, G., Petsche, H., Sterc, J., Stumpf, C.H., 1967. Septum cell activity in the rabbit under reticular stimulation. Brain Res. 5, 508–510. Gogolák, G., Stumpf, C.H., Petsche, H., Sterc, J., 1968. The firing pattern of septal neurons and the form of the hippocampal theta wave. Brain Res. 7, 201–207. Gong, J., 1984. Direct connections between hypothalamus and lumbar spinal cord in rabbits. Sci. Sinica Ser. B Chem. Biol. Agric. Med. Earth Sci. 27, 789–799. Gonzalo-Ruiz, A., Alonso, A., Sanz, J.M., Llinas, R., 1992a. A dopaminergic projection to the rat mammillary nuclei demonstrated by retrograde transport of wheat germ agglutinin-horseradish peroxidase and tyrosine hydroxylase immunohistochemistry. J. Comp. Neurol. 321, 300–311. Gonzalo-Ruiz, A., Alonso, A., Sanz, J.M., Llinás, R.R., 1992b. Afferent projections to the mammillary complex of the rat, with special reference to those from surrounding hypothalamic regions. J. Comp. Neurol. 321, 277–299. Gonzalo-Ruiz, A., Morte, L., Flecha, J.M., Sanz, J.M., 1999. Neurotransmitter characteristics of neurons projecting to the supramammillary nucleus of the rat. Anat. Embryol. 200, 377–392. Gray, J.A., 1970. Sodium amobarbital, the hippocampal theta rhythm and the partial reinforcement extinction effect. Psychol. Rev. 77, 465–480. Gray, J.A., 1971. Medial septal lesions, hippocampal theta rhythm and the control of vibrissal movement in the freely moving rat. Electroencephalogr. Clin. Neurophysiol. 30, 189–197. Gray, J.A., 1976. The behavioural inhibition system: a possible substrate for anxiety. In: Feldman, M.P., Broadhurst, A.M. (Eds.), Theoretical and Experimental Bases of Behaviour Modification. Wiley, London, pp. 3–41. Gray, J.A., 1982. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-hippocampal System. Oxford University Press, Oxford. 161 Gray, J.A., Ball, G.G., 1970. Frequency-specific relation between hippocampal theta rhythm, behavior and amobarbital action. Science 168, 1246–1248. Gray, J.A., McNaughton, N., 1983. Comparison between the behavioural effect of septal and hippocampal lesions: a review. Neurosci. Biobehav. Rev. 7, 119–188. Gray, J.A., McNaughton, N., 2000. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-hippocampal System. Oxford University Press, Oxford. Green, J.S., Arduini, A., 1954. Hippocampal electrical activity in arousal. J. Neurophysiol. 17, 533–557. Greenwood, R.S., Godar, S.E., Reaves, T.A., Hayward, J.N., 1981. Cholecystokinin in hippocampal pathways. J. Comp. Neurol. 203, 335–350. Grove, E.A., 1988. Neural associations of the substantia innominata in the rat: afferent connections. J. Comp. Neurol. 277, 315–346. Gundlah, C., Pecins-Thompson, M., Schutzer, W.E., Bethea, C.L., 1999. Ovarian steroid effects on serotonin 1A, 2A and 2C receptor mRNA in macaque hypothalamus. Mol. Brain Res. 63, 325–339. Haefely, W., 1990a. The GABAa–benzodiazepine receptor: biology and pharmacology. In: Burrows, G.D., Roth, M., Noyes, R. (Eds.), Handbook of Anxiety: The Neurobiology of Anxiety. Elsevier, pp. 165– 187. Haefely, W., 1991. Pharmacology of anxiety. Eur. Neuropsychopharmacol. 1, 89–95. Haefely, W., 1992. Ligands of the GABAa receptor-associated benzodiazepine receptor. Neurosci. Facts 3, 69–70. Haefely, W.E., 1990b. The GABAa–benzodiazepine receptor complex and anxiety. In: Sartorius, N., Andreoli, V., Cassano, G., Eisenberg, L., Kielholz, P., Pancheri, P., Racagni, G. (Eds.), Anxiety: Psychobiological and Clinical Perspectives. Hemisphere Publishing Corporation, New York, pp. 23–36. Haglund, L., Swanson, L.W., Köhler, C., 1984. The projection of the supramammillary nucleus to the hippocampal formation: an immunohistochemical and anterograde transport study with the lectin PHA-L in the rat. J. Comp. Neurol. 229, 171–185. Haines, D.E., May, P.J., Dietrichs, E., 1990. Neuronal connections between the cerebellar nuclei and hypothalamus in Macaca fascicularis: cerebello-visceral circuits. J. Comp. Neurol. 299, 106–122. Hanada, Y., Hallworth, N.E., Szgatti, T.L., Scarlett, D., Bland, B.H., 1999. Distribution and analysis of hippocampal theta-related cells in the pontine region of the urethane-anesthetized rat. Hippocampus 9, 288–302. Harley, C.W., Martin, G.M., 1999. Open field motor patterns and object marking, but not object sniffing, are altered by ibotenate lesions of the hippocampus. Neurobiol. Learn. Mem. 72, 202–214. Hausheer-Zarmakupi, Z., Wolfer, D.P., Leisinger-Trigona, M.C., Lipp, H.P., 1996. Selective breeding for extremes in open-field activity of mice entails a differentiation of hippocampal mossy fibers. Behav. Genet. 26, 167–176. Hayakawa, T., Ito, H., Zyo, K., 1993. Neuroanatomical study of afferent projections to the supramammillary nucleus of the rat. Anat. Embryol. 188, 139–148. Hayakawa, T., Zyo, K., 1994. Fine structure of the supramammillary nucleus of the rat: analysis of the ultrastructural character of dopaminergic neurons. J. Comp. Neurol. 346, 127–136. Heidbreder, C.A., Groenewegen, H.J., 2003. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci. Biobehav. Rev. 27, 555– 579. Henderson, Z., Morris, N.P., Grimwood, P., Fiddler, G., Yang, H.W., Appenteng, K., 2001. Morphology of local axon collaterals of electrophysiologically characterised neurons in the rat medial septal/diagonal band complex. J. Comp. Neurol. 430, 410–432. Herman, J.P., Dolgas, C.M., Carlson, S.L., 1998. Ventral subiculum regulates hypothalamo-pituitary–adrenocortical and behavioural responses to cognitive stressors. Neuroscience 86, 449–459. Ikemoto, S., Witkin, B.M., Morales, M., 2003. Rewarding injections of the cholinergic agonist carbachol into the ventral tegmental area induce 162 W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 locomotion and c-Fos expression in the retrosplenial area and supramammillary nucleus. Brain Res. 969, 78–87. Ino, T., Sugimoto, T., Kaneko, T., Kamiya, H., Mizuno, N., 1988. The supramammillary region of the cat sends substance P-like immunoreactive axons to the hippocampal formation and the entorhinal cortex. Neurosci. Lett. 90, 259–264. Ito, M., Doya, K., Shirao, T., Sekino, Y., 2003. Spatial distribution of fos-positive neurons in the supramammillary nucleus and the hippocampus of rats placed in a novel environment. Soc. Neurosci. Abstr. 717.13 . James, D.T.D., McNaughton, N., Rawlins, J.N.P., Feldon, J., Gray, J.A., 1977. Septal driving of hippocampal theta rhythm as a function of frequency in the free-moving male rat. Neuroscience 2, 1007– 1017. Jarrard, L.E., Becker, J.T., 1977. The effects of selective hippocampal lesions on DRL behavior in rats. Behav. Biol. 21, 393–404. Jarrard, L.E., Okaichi, H., Steward, O., Goldschmidt, R.B., 1984. On the role of hippocampal connections in the performance of place and cue tasks: comparisons with damage to hippocampus. Behav. Neurosci. 98, 946–954. Jeffery, K.J., Donnett, J.G., O’Keefe, J., 1995. Medial septal control of theta-correlated unit firing in the entorhinal cortex of awake rats. Neuroreport 6, 2166–2170. Jiang, F., Khanna, S., 2004. Reticular stimulation evokes suppression of ca1 synaptic responses and generation of theta through separate mechanisms. Eur. J. Neurosci. 19, 295–308. Jung, R., Kornmüller, A.E., 1938. Eine Methodik der Ableitung lokalisierter Potentialschwankungen aus subcorticalen Hirngebieten. Archiv für Psychiatrie 109, 1–30. Kataoka, Y., Shibata, K., Gomita, Y., Ueki, S., 1982. The mammillary body is a potential site of antianxiety action of benzodiazepines. Brain Res. 241, 374–377. Kirk, I.J., 1993. Supramammillary control of the frequency of hippocampal rhythmical slow-wave activity (theta). Ph.D. Thesis. University of Otago, Dunedin, New Zealand. Kirk, I.J., 1997. Supramammillary neural discharge patterns and hippocampal EEG. Brain Res. Bull. 42, 23–26. Kirk, I.J., 1998. Frequency modulation of hippocampal theta by the supramammillary nucleus, and other hypothalamo-hippocampal interactions: mechanisms and functional implications. Neurosci. Biobehav. Rev. 22, 291–302. Kirk, I.J., Mackay, J.C., 2003. The role of theta-range oscillations in synchronising and integrating activity in distributed mnemonic networks. Cortex 39, 993–1008. Kirk, I.J., McNaughton, N., 1991. Supramammillary cell firing and hippocampal rhythmical slow activity. Neuroreport 2, 723–725. Kirk, I.J., McNaughton, N., 1993. Mapping the differential effects of procaine on frequency and amplitude of reticularly elicited hippocampal rhythmical slow activity. Hippocampus 3, 517–526. Kirk, I.J., Oddie, S.D., Konopacki, J., Bland, B.H., 1996. Evidence for differential control of posterior hypothalamic, supramammillary, and medial mammillary theta-related cellular discharge by ascending and descending pathways. J. Neurosci. 16, 5547–5554. Kiss, J., Borhegyi, Z., Csaky, A., Szeiffert, G., Leranth, C., 1997a. Parvalbumin-containing cells of the angular portion of the vertical limb terminate on calbindin immunoreactive neurons located at the border between the lateral and medial septum of the rat. Exp. Brain Res. 113, 48–56. Kiss, J., Csaki, A., Bokor, H., Kocsis, K., Kocsis, B., 2002. Possible glutamatergic/aspartatergic projections to the supramammillary nucleus and their origins in the rat studied by selective [3H]D-aspartate labelling and immunocytochemistry. Neuroscience 111, 671–691. Kiss, J., Csáki, A., Bokor, H., Shanabrough, M., Leranth, C., 2000. The supramammillo-hippocampal and supramammillo-septal glutamatergic/aspartatergic projections in the rat: a combined [3H]D-aspartate autoradiographic and immunohistochemical study. Neuroscience 97, 657–669. Kiss, J., Magloczky, Z., Somogyi, J., Freund, T.F., 1997b. Distribution of calretinin-containing neurons relative to other neurochemically identified cell types in the medial septum of the rat. Neuroscience 78, 399–410. Kiyama, K., Shiosaka, S., Takami, K., Tateishi, K., Ashimura, E., Hamaoka, T., Tohyama, K., 1984a. CCK pathway from supramammillary region to the nucleus anterior ventralis thalami of the young rats. Peptides 5, 889–893. Kiyama, K., Shiosaka, S., Tateishi, K., Hashimura, E., Hamaoka, T., Tohyama, K., 1984b. Cholecystokinin-8-like immunoreactive neuron pathway from the supramammillary region to the ventral tegmental nucleus of Gudden of the rat. Brain Res. 304, 397–400. Kiyohara, T., Miyata, S., Nakamura, T., Shido, O., Nakashima, T., Shibata, M., 1995. Differences in fos expression in the rat brains between cold and warm ambient exposures. Brain Res. Bull. 38, 193–201. Kocsis, B., Di Prisco, G.V., Vertes, R.P., 2001. Theta synchronization in the limbic system: the role of Gudden’s tegmental nuclei. Eur. J. Neurosci. 13, 381–388. Kocsis, B., Kiss, J., Csaky, A., Halasz, B., 2003. Location of putative glutamatergic neurons projecting to the medial preoptic area of the rat hypothalamus. Brain Res. Bull. 61, 459–468. Kocsis, B., Vertes, R.P., 1992. Dorsal raphe neurons: synchronous discharge with the theta rhythm of the hippocampus in the freely behaving rat. J. Neurophysiol. 68, 1463–1467. Kocsis, B., Vertes, R.P., 1994. Characterization of neurons of the supramammillary nucleus and mammillary body that discharge rhythmically with the hippocampal theta rhythm in the rat. J. Neurosci. 14, 7040– 7052. Kocsis, B., Vertes, R.P., 1996. Midbrain raphe cell firing and hippocampal theta rhythm in urethane-anaesthetized rats. Neuroreport 7, 2867– 2872. Kocsis, B., Vertes, R.P., 1997. Phase relations of rhythmic neuronal firing in the supramammillary nucleus and mammillary body to the hippocampal theta activity in urethane anesthetized rats. Hippocampus 7, 204–214. Krayniak, P.F., Weiner, S., Siegel, A., 1980. An analysis of the efferent connections of the septal area in the cat. Brain Res. 189, k15–k29. Kveim, O., Setekleiv, J., Kaada, B.R., 1964. Differential effects of hippocampal lesions on maze and passive avoidance learning in rats. Exp. Neurol. 9, 59–72. Lamour, Y., Dutar, P., Jobert, A., 1984. Septo-hippocampal and other medial septum-diagonal band neurons: electrophysiological and pharmacological properties. Brain Res. 209, 227–239. Lantos, T.A., Görcs, T.J., Palkovits, M., 1995. Immunohistochemical mapping of neuropeptides in the premammillary region of the hypothalamus in rats. Brain Res. Rev. 20, 209–249. LeDoux, J.E., 1993a. Emotional memory systems in the brain. Behav. Brain Res. 58, 69–79. LeDoux, J.E., 1993b. Emotional memory: in search of systems and synapses. Ann. NY Acad. Sci. 702, 149–157. LeDoux, J.E., 1995. Emotion: clues from the brain. Annu. Rev. Psychol. 46, 209–235. LeDoux, J.E., Iwata, J., Cicchetti, P., Reis, D.J., 1988. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditional fear. J. Neurosci. 8 (7), 2517–2529. Lee, M.G., Chrobak, J.J., Sik, A., Wiley, R.G., Buzsáki, G., 1994. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience 62, 1033–1047. Leranth, C., Carpi, D., Buzsáki, G., Kiss, J., 1999. The entorhino-septosupramammillary nucleus connection in the rat: morphological basis of a feedback mechanism regulating hippocampal theta rhythm. Neuroscience 88, 701–718. Leranth, C., Deller, T., Buzsáki, G., 1992. Intraseptal connections redefined: lack of a lateral septum to medial septum path. Brain Res. 583, 1–11. Leranth, C., Frotscher, M., 1989. Organization of the septal region in the rat brain: cholinergic–GABAergic interconnections and the termination of hippocampo-septal fibers. J. Comp. Neurol. 289, 304–314. W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 Leranth, C., Kiss, J., 1996. A population of supramammillary area calretinin neurons terminating on medial septal area cholinergic and lateral septal area calbindin-containing cells are aspartate/glutamatergic. J. Neurosci. 16, 7699–7710. Leranth, C., Nitsch, R., 1994. Morphological evidence that hypothalamic substance P-containing afferents are capable of filtering the signal flow in the monkey hippocampal formation. J. Neurosci. 14, 4079– 4094. Leranth, C., Shanabrough, M., 2001. Supramammillary area mediates subcortical estrogenic action on hippocampal synaptic plasticity. Exp. Neurol. 167, 445–450. Leung, L.S., 1998. Generation of theta and gamma rhythms in the hippocampus. Neurosci. Biobehav. Rev. 22, 275–290. Leung, L.S., Borst, J.G.G., 1987. Electrical activity of the cingulate cortex. I. Generating mechanisms and relations to behaviour. Brain Res. 407, 68–80. Lin, S.H., Miyata, S., Matsunaga, W., Kawarabayashi, T., Nakashima, T., Kiyohara, T., 1998. Metabolic mapping of the brain in pregnant, parturient and lactating rats using fos immunohistochemistry. Brain Res. 787, 226–236. Maglóczky, Z., Acsády, L., Freund, T.F., 1994. Principal cells are the postsynaptic targets of supramammillary afferents in the hippocampus of the rat. Hippocampus 4, 322–334. Manning, F.J., McDonough, J.H., 1974. Reinforcement omission, noncontingent reinforcement, and limbic lesions in rats. Behav. Biol. 11, 327–338. McCarthy, M.M., Felzenberg, E., Robbins, A., Pfaff, D.W., SchwartzGiblin, S., 1995. Infusions of diazepam and allopregnanolone into the midbrain central gray facilitate open field behaviour and sexual receptivity in female rats. Horm. Behav. 29, 279–295. McDonald, R.J., Hong, N.S., 2000. Rats with hippocampal damage are impaired on place learning in the water task when overtrained under constrained conditions. Hippocampus 10, 153–161. McEwen, B.S., 1994. Corticosteroids and hippocampal plasticity. Ann. NY Acad. Sci. 746, 134–144. McEwen, B.S., 1999. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 22, 105–122. McEwen, B.S., Weiss, J.M., Schwartz, L.S., 1969. Uptake of corticosterone by rat brain and its concentration by certain limbic structures. Brain Res. 16, 227–241. McNaughton, N., 1977. Exploration, frustration and the electrophysiology of the septohippocampal theta system in the rat. Southampton University. McNaughton, N., Coop, C.F., 1991. Neurochemically dissimilar anxiolytic drugs have common effects on hippocampal rhythmic slow activity. Neuropharmacology 30, 855–863. McNaughton, N., Logan, B., Panickar, K.S., Kirk, I.J., Pan, W.-X., Brown, N.T., Heenan, A., 1995. Contribution of synapses in the medial supramammillary nucleus to the frequency of hippocampal theta rhythm in freely moving rats. Hippocampus 5, 534–545. McNaughton, N., Morris, R.G.M., 1987. Chlordiazepoxide, an anxiolytic benzodiazepine, impairs place navigation in rats. Behav. Brain Res. 24, 39–46. McNaughton, N., Sedgwick, E.M., 1978. Reticular stimulation and hippocampal theta rhythm in rats: effects of drugs. Neuroscience 2, 629–632. Miller, C.R., Elkins, R.L., Fraser, J., Peacock, L.J., Hobbs, S.H., 1975. Taste aversion and passive avoidance in rats with hippocampal lesions. Physiol. Psychol. 3, 123–126. Miller, R., 1989. Cortico-hippocampal interplay: self-organizing phaselocked loops for indexing memory. Psychobiology 17, 115–128. Miller, R., 1991. Cortico-hippocampal Interplay and the Representation of Contexts in the Brain. Springer-Verlag, Berlin. Miyata, S., Ishiyama, M., Shido, O., Nakashima, T., Shibata, M., Kiyohara, T., 1995. Central mechanism of neural activation with cold acclimation of rats using Fos immunohistochemistry. Neurosci. Res. 22, 209–218. Mizumori, S.J.Y., McNaughton, B.L., Barnes, C.A., 1989. A comparison of supramammillary and medial septal influences on hippocampal field potentials and single-unit activity. J. Neurophysiol. 61, 15–31. 163 Morris, R.G.M., 1984. Development of a water-maze procedure for the study of spatial learning in the rat. J. Neurosci. Meth. 11, 47–60. Morris, R.G.M., Garrud, P., Rawlins, J.N.P., O’Keefe, J., 1982. Place navigation impaired in rats with hippocampal lesions. Nature (London) 297, 681–683. Morris, R.G.M., Hagan, J.J., 1983. Hippocampal electrical activity and ballistic movement. In: Seifert, W. (Ed.), Neurobiology of the Hippocampus. Academic press, London, pp. 321–331. Moser, M.B., Moser, E.I., Forrest, E., Andersen, P., Morris, R.G.M., 1995. Spatial learning with a minislab in the dorsal hippocampus. Proc. Natl. Acad. Sci. U.S.A. 92, 9697–9701. Nakanishi, K., Saito, H., Abe, K., 2001. The supramammillary nucleus contributes to associative EPSP-spike potentiation in the rat dentate gyrus in vivo. Eur. J. Neurosci. 13, 793–800. Nitsch, R., Leranth, C., 1993. Calretinin immunoreactivity in the monkey hippocampal formation. II. Intrinsic gabaergic and hypothalamic nongabaergic systems: an experimental tracing and co-existence study. Neuroscience 55, 797–812. Nitsch, R., Leranth, C., 1996. GABAergic neurons in the rat dentate gyrus are innervated by subcortical calretinin-containing afferents. J. Comp. Neurol. 364, 425–438. Numan, Seroogy, K., 1999. Expression of trkB and trkC mRNAs by adult midbrain dopamine neurons: a double-label in situ hybridization study. J. Comp. Neurol. 403, 295–308. Nunez, A., Garcia Austt, E., Buno, W., 1990. Synaptic contributions to theta rhythm genesis in rat CA1–CA3 hippocampal pyramidal neurons in vivo. Brain Res. 533, 176–179. Nunez, A., Garcia Austt, E., Buno, W.J., 1987. Intracellular theta-rhythm generation in identified hippocampal pyramids. Brain Res. 416, 289– 300. O’Keefe, J., Nadel, L., 1978. The Hippocampus as a Cognitive Map. Clarendon Press, Oxford. O’Keefe, J., Recce, M.L., 1993. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3, 317–330. Oddie, S.D., Bland, B.H., 1998. Hippocampal formation theta activity and movement selection. Neurosci. Biobehav. Rev. 22, 221–231. Oddie, S.D., Bland, B.H., Colom, L.V., Vertes, R.P., 1994. The midline posterior hypothalamic region comprises a critical part of the ascending brainstem hippocampal synchronizing pathway. Hippocampus 4, 454– 473. Oddie, S.D., Stefanek, W., Kirk, I.J., Bland, B.H., 1996. Intraseptal procaine abolishes hypothalamic stimulation-induced wheelrunning and hippocampal theta field activity in rats. J. Neurosci. 16, 1948–1956. Olivier, B., Olivier-Aardema, R., Wiepkema, P.R., 1983. Effect of anterior hypothalamic and mammillary area lesions on territorial aggressive behaviour in male rats. Behav. Brain Res. 9, 59–81. Ottersen, O.P., 1980. Afferent connections to the amygdaloid complex of the rat and cat. II. Afferents from the hypothalamus and the basal telencephalon. J. Comp. Neurol. 194, 267–289. Pan, W.-X., 2000. The role of the medial supramammillary nucleus in hippocampal theta activity and behavior in rats. Ph.D. Thesis. University of Otago. Pan, W.-X., Hyland, B., 2001. The activity of dopamine neurons in rats during learning of classical conditioned stimulus-reward association. Int. J. Neurosci. 109, 208. Pan, W.-X., McNaughton, N., 1996. The effects of injection of procaine and chlordiazepoxide into the supramammillary nucleus on the frequency of hippocampal theta rhythm in free moving rats. Int. J. Neurosci. 84, 280. Pan, W.-X., McNaughton, N., 1997. The medial supramammillary nucleus, spatial learning and the frequency of hippocampal theta activity. Brain Res. 764, 101–108. Pan, W.-X., McNaughton, N., 2002. The role of the medial supramammillary nucleus in the control of hippocampal theta activity and behaviour in rats. Eur. J. Neurosci. 16, 1797–1809. 164 W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 Papez, J.W., 1937. A proposed mechanism of emotion. Arch. Neurol. Psychiatr. 38, 725–743. Parmeggiani, P.L., Azzaroni, A., Lenzi, P., 1971. On the functional significance of the circuit of Papez. Brain Res. 30, 357–374. Parmeggiani, P.L., Lenzi, P.L., Azzaroni, A., 1974. Transfer of the hippocampal output by the anterior thalamic nuclei. Brain Res. 67, 269–278. Pasquier, D.A., Reinoso-Suarez, F., 1978a. Direct projections from the hypothalamus to hippocampus in the rat demonstrated by retrograde transport of horseradish peroxidase. Brain Res. 108, 165– 169. Pasquier, D.A., Reinoso-Suarez, F., 1978b. The topographic organization of hypothalamic and brain stem projections to the hippocampus. Brain Res. Bull. 3, 373–389. Paxinos, G., Watson, C., 1998. The Rat Brain in Stereotaxic Coordinates. Academic Press, Sydney. Petsche, H., Stumpf, C., 1960. Topographic and toposcopic study of origin and spread of the regular synchronised arousal pattern in the rabbit. Electroencephalogr. Clin. Neurophysiol. 12, 589–600. Petsche, H., Stumpf, C., Gogolak, G., 1962. The significance of the rabbit’s septum as a relay station between the midbrain and the hippocampus. I. The control of hippocampus arousal activity by the septum cells. Electroencephalogr. Clin. Neurophysiol. 14, 202–211. Phillips, R.G., LeDoux, J.E., 1992. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 106, 274–285. Phillips, R.G., LeDoux, J.E., 1994. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn. Mem. 1, 34–44. Phillips, R.G., LeDoux, J.E., 1995. Lesions of the fornix but not the entorhinal or perirhinal cortex interfere with contextual fear conditioning. J. Neurosci. 15, 5308–5315. Phillipson, O.T., 1979. The cytoarchitecture of the interfacicular nucleus and ventral tegmental area of Tsai in the rat. J. Comp. Neurol. 187, 85– 98. Price, J.L., Amaral, D.G., 1981. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J. Neurosci. 1, 1242–1259. Rabe, A., Haddad, R.K., 1968. Effect of selective hippocampal lesions in the rat on acquisition, performance, and extinction of bar pressing on a fixed ratio schedule. Exp. Brain Res. 5, 259–266. Rawlins, J.N.P., Feldon, J., Gray, J.A., 1979. Septo-hippocampal connections and the hippocampal theta rhythm. Exp. Brain Res. 37, 49–63. Richmond, M.A., Yee, B.K., Pouzet, B., Veenman, L., Rawlins, J.N.P., Feldon, J., Bannerman, D.M., 1999. Dissociating context and space within the hippocampus: effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behav. Neurosci. 113, 1189–1203. Risold, P.Y., Swanson, L.W., 1996. Structural evidence for functional domains in the rat hippocampus. Science 272, 1484–1486. Risold, P.Y., Swanson, L.W., 1997. Connections of the rat lateral septal complex. Brain Res. Rev. 24, 115–195. Ritter, S., Dinh, T.T., 1988. Capsaicin-induced neuronal degeneration: silver impregnation of cell bodies, axons, and terminals in the central nervous system of the adult rat. J. Comp. Neurol. 271, 79–90. Robinson, T.E., Vanderwolf, C.H., 1978. Electrical stimulation of the brain stem in freely moving rats. II. Effects of hippocampal and neocortical electrical activity and relations to behavior. Exp. Neurol. 61, 485–515. Roozendaal, B., McGaugh, J.L., 1997. Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. Eur. J. Neurosci. 9, 76–83. Roozendaal, B., Portillo-Marquez, G., McGaugh, J.L., 1996. Basolateral amygdala lesions block glucocorticoid-induced modulation of memory for spatial learning. Behav. Neurosci. 110, 1074–1083. Roozendaal, B., Sapolsky, R.M., McGaugh, J.L., 1998. Basolateral amygdala lesions block the disruptive effects of long-term adrenalectomy on spatial memory. Neuroscience 84, 453–465. Rossi-Arnaud, C., Ammassari-Teule, M., 1992. Modifications of open field and novelty behaviors by hippocampal and amygdaloid lesions in two inbred strains of mice: lack of strain–lesion interactions. Behav. Process. 27, 155–164. Routtenberg, A., Taub, F., 1973. Hippocampus and superior colliculus: congruent EEG activity demonstrated by a simple measure. Behav. Biol. 8, 801–805. Sailer, S., Stumpf, C., 1957. Beeinflussbarkeit der Rhinencephalen tatigkeit des Kaninchens. Arch. Exp. Path. Pharmak. 231, 63–77. Sainsbury, R.S., 1998. Hippocampal theta: a sensory-inhibition theory of function. Neurosci. Biobehav. Rev. 22, 237–241. Saji, M., Kobayashi, S., Ohno, K., Sekino, Y., 2000. Interruption of supramammillohippocampal afferents prevents the genesis and spread of limbic seizures in the hippocampus via a disinhibition mechanism. Neuroscience 97, 437–445. Sakanaka, M., Shiosaka, S., Takagi, H., Senba, E., Takatsuki, K., Inagaki, S., Yabuuchi, H., Matsuzaki, T., Tohyama, M., 1980. Topographic organization of the projection from the forebrain areas to the hippocampal formation of the rat. Neurosci. Lett. 20, 253–258. Sandner, G., Di, S.G., Rocha, B., Angst, M.J., 1992. C-fos immunoreactivity in the brain following unilateral electrical stimulation of the dorsal periaqueductal gray in freely moving rats. Brain Res. 573, 276–283. Santin, L.J., Aguirre, J.A., Rubio, S., Begega, A., Miranda, R., Arias, J.L., 2003. C-fos expression in supramammillary and medial mammillary nuclei following spatial reference and working memory tasks. Physiol. Behav. 78, 733–739. Saper, C.B., Swanson, L.W., Cowan, W.M., 1976. The efferent connections of the ventromedial nucleus of the hypothalamus of the rat. J. Comp. Neurol. 169, 442–490. Schmitt, M.L., Graeff, F.G., Carobrez, A.D.P., 1990. Anxiolytic effect of kynurenic acid microinjected into the dorsal periaqueductal gray matter of rats placed in the elevated plus-maze test. Braz. J. Med. Biol. Res. 23, 677–679. Schmitt, M.L., Graeff, F.G., Carobrez, A.P., 2001. Anxiolytic effect of hynurenic acid microinjected into the dorsal periaqueductal gray matter of rats placed in the elevated plus maze test. Braz. J. Med. Biol. Res. 23, 677–679. Segal, M., 1977. The effects of brainstem priming stimulation on interhemispheric hippocampal responses in the awake rat. Exp. Brain Res. 28, 529–541. Segal, M., 1979. A potent inhibitory monosynaptic hypothalamo-hippocampal connection. Brain Res. 162, 137–141. Segal, M., Bloom, F.E., 1974. The action of norepinephrine in the rat hippocampus. I. Iontophoretic studies. Brain Res. 72, 79–97. Segal, M., Bloom, F.E., 1976. The action of norepinephrine in the rat hippocampus. IV. The effects of locus coeruleus stimulation on evoked hippocampal unit activity. Brain Res. 107, 513–525. Segal, M., Landis, S.C., 1974. Afferents to the hippocampus of the rat studied with the method of retrograde transport of horseradish peroxidase. Brain Res. 78, 1–15. Seroogy, K., Tsuruo, Y., Hökfelt, T., Walsh, J., Fahrenkrug, J., Emson, P.C., Goldstein, M., 1988. Further analysis of presence of peptides in dopamine neurons. Cholecystokinin, peptide histidine–isoleucine/ vasoactive intestinal polypeptide and substance P in rat supramammillary region and mesencephalon. Exp. Brain Res. 72, 523–534. Shahidi, S., Motamedi, F., Bakeshloo, A.S., Taleghani, B.K., 2004. The effect of reversible inactivation of the supra-mammillary nucleus on passive avoidance learning in rats. Behav. Brain Res. 152, 81–87. Shepard, P.D., Mihailoff, G.A., German, D.C., 1988. Anatomical and electrophysiological characterization of presumed dopamine-containing neurons within the supramammillary region of the rat. Brain Res. Bull. 20, 307–314. W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 Shibata, H., 1987. Ascending projections to the mammillary nuclei in the rat: a study using retrograde and anterograde transport of wheat germ agglutin conjugated to horseradish peroxidase. J. Comp. Neurol. 264, 205–215. Shibata, H., Suzuki, T., Matsushita, M., 1986. Afferent projections to the interpeduncular nucleus in the rat, as studied by retrograde and anterograde transport of wheat germ agglutinin conjugated to horseradish peroxidase. J. Comp. Neurol. 248, 272–284. Silveira, M.C.L., Sandner, G., Di Scala, G., Graeff, F.G., 1995. C-fos immunoreactivity in the brain following electrical or chemical stimulation of the medial hypothalamus of freely moving rats. Brain Res. 674, 265–274. Silveira, M.C.L., Sandner, G., Graeff, F.G., 1993. Induction of fos immunoreactivity in the brain by exposure to the elevated plus-maze. Behav. Brain Res. 56, 115–118. Sinnamon, H.M., 1984. Forelimb and hindlimb stepping by the anesthetized rat elicited by electrical stimulation of the diencephalon and mesencephalon. Physiol. Behav. 33, 191–199. Smythe, J.W., Christie, B.R., Colom, L.V., Lawson, V.H., Bland, B.H., 1991. Hippocampal theta field activity and theta-on/theta-off cell discharges are controlled by an ascending hypothalamo-septal pathway. J. Neurosci. 11, 2241–2248. Smythe, J.W., Colom, L.V., Bland, B.H., 1992. The extrinsic modulation of hippocampal theta depends on the coactivation of cholinergic and GABA-ergic medial septal inputs. Neurosci. Biobehav. Rev. 16, 289– 308. Souza, M.M.D.S., Schenberg, L.C., Carobrez, A.D.P., 1998. NMDAcoupled periaqueductal gray glycine receptors modulate anxioselective drug effects on plus-maze performance. Behav. Brain Res. 90, 157–165. Stanfield, B.B., Cowan, W.M., 1984. An EM autoradiographic study of the hypothalamo-hippocampal projection. Brain Res. 309, 299–307. Stanfield, B.B., Wyss, J.M., Cowan, W.M., 1980. The projection of the supramammillary region upon the dentate gyrus in normal and reeler mice. Brain Res. 198, 196–203. Stefanski, R., Palejko, W., Bidzinski, A., Kostowski, W., Plaznik, A., 1993. Serotonergic innervation of the hippocampus and nucleus accumbens septi and the anxiolytic-like action of midazolam and 5-HT1A receptor agonists. Neuropharmacology 32, 977–985. Stumpf, C., 1965. Drug action on the electrical activity of the hippocampus. Int. Rev. Neurobiol. 8, 77–138. Stumpf, C., Petsche, H., Gogolak, G., 1962. The significance of the rabbit’s septum as a relay station between the midbrain and hippocampus. II. The differential influence of drugs upon the septal cell firing and the hippocampal theta activity. Electroencephalogr. Clin. Neurophysiol. 14, 212–219. Swanson, L.W., 1976. An autoradiographic study of the efferent connections of the preoptic region in the rat. J. Comp. Neurol. 167, 227–256. Swanson, L.W., 1982. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res. Bull. 9, 321–353. Swanson, L.W., 1998. Brain Maps: Structure of the Rat Brain. Elsevier, Amsterdam. Swanson, L.W., Cowan, W.M., 1975. The efferent connections of the suprachiasmatic nucleus of the hypothalamus. J. Comp. Neurol. 160, 1–12. Swanson, L.W., Cowan, W.M., 1979. The connections of the septal region in the rat. J. Comp. Neurol. 186, 621–655. Swanson, L.W., Petrovich, G.D., 1998. What is the amygdala? Trends Neurosci. 21, 323–331. Sziklas, V., Petrides, M., 1993. Memory impairments following lesions to the mammillary region of the rat. Eur. J. Neurosci. 5, 525–540. Sziklas, V., Petrides, M., 1998. Memory and the region of the mammillary bodies. Prog. Neurobiol. 54, 55–70. Sziklas, V., Petrides, M., Jackson, P., 1995. Selectivity of spatial learning impairment in rats with lesions of the mammillary region. Soc. Neurosci. Abstr. 21, 1938. 165 Sziklas, V., Petrides, M., Leri, F., 1996. The effects of lesions to the mammillary region and the hippocampus on conditional associative learning by rats. Eur. J. Neurosci. 8, 106–115. Thinschmidt, J.S., 1993. The supramammillary nucleus: does it play a role in the mediation of hippocampal theta rhythm? MA Thesis. Florida Atlantic University. Thinschmidt, J.S., Kinney, G.G., Kocsis, B., 1995. The supramammillary nucleus: is it necessary for the mediation of hippocampal theta rhythm? Neuroscience 67, 301–312. Tonkiss, J., Morris, R.G.M., Rawlins, J.N.P., 1988. Intra-ventricular infusion of the NMDA antagonist AP5 impairs performance on a non-spatial operant DRL task in the rat. Exp. Brain Res. 73, 181–188. Tóth, K., Freund, T.F., Miles, R., 1997. Disinhibition of rat hippocampal pyramidal cells by GABAergic afferents from the septum. J. Physiol. (Lond.) 500, 463–474. Tömböl, T., Petsche, H., 1969. The histological organisation of the pacemaker for the hippocampal theta rhythm in the rabbit. Brain Res. 12, 414–426. Uno, H., Tarara, R., Else, J.G., Suleman, M.A., Sapolsky, R.M., 1989. Hippocampal damage associated with prolonged and fatal stress in primates. J. Neurosci. 9, 1705–1711. Vann, S.D., Brown, M.W., Aggleton, J.P., 2000. Fos expression in the rostral thalamic nuclei and associated cortical regions in response to different spatial memory tests. Neuroscience 101, 983–991. Vertes, R.P., 1980. Brain stem activation of the hippocampus: a role for the magnocellular reticular formation and the MLF. Electroencephalogr. Clin. Neurophysiol. 50, 48–58. Vertes, R.P., 1981. An analysis of ascending brain stem systems involved in hippocampal synchronization and desynchronization. J. Neurophysiol. 46, 1140–1159. Vertes, R.P., 1982. Brain stem generation of the hippocampal EEG. Prog. Neurobiol. 19, 159–186. Vertes, R.P., 1986. Brainstem modulation of the hippocampus: anatomy, physiology and significance. In: Isaacson, R.L., Pribram, K.H. (Eds.), The Hippocampus. Plenum Press, New York, pp. 41–75. Vertes, R.P., 1988. Brainstem afferents to the basal forebrain in the rat. Neuroscience 24, 907–935. Vertes, R.P., 1992. PHA-L analysis of projections from the supramammillary nucleus in the rat. J. Comp. Neurol. 326, 595–622. Vertes, R.P., Fortin, W.J., Crane, A.M., 1999. Projections of the median raphe nucleus in the rat. J. Comp. Neurol. 407, 555–582. Vertes, R.P., Kocsis, B., 1997. Brainstem–diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience 81, 893–926. Vertes, R.P., Martin, G.F., 1988. Autoradiographic analysis of ascending projections from the pontine and mesencephalic reticular formation and the median raphe nucleus in the rat. J. Comp. Neurol. 275, 511–541. Vertes, R.P., McKenna, J.T., 2000. Collateral projections from the supramammillary nucleus to the medial septum and hippocampus. Synapse 38, 281–293. Vinogradova, O.S., 1995. Expression, control, and probable functional significance of the neuronal theta-rhythm. Prog. Neurobiol. 45, 523– 583. Wetzel, W., Ott, T., Matthies, H., 1977a. Hippocampal rhythmic slow activity (theta) and behavior elicited by medial septal stimulation in rats. Behav. Biol. 19, 534–542. Wetzel, W., Ott, T., Matthies, H., 1977b. Post-training hippocampal rhythmic slow activity (theta) elicited by septal stimulation improves memory consolidation in rats. Behav. Biol. 21, 32–40. Whishaw, I.Q., Bland, B.H., Vanderwolf, C.H., 1972. Hippocampal activity, behavior, self-stimulation, and heart rate during electrical stimulation of the lateral hypothalamus. J. Comp. Physiol. Psychol. 79, 115–127. Whishaw, I.Q., Vanderwolf, C.H., 1971. Hippocampal EEG and behavior: effects of variation in body temperature and relation of EEG to vibrissae movement, swimming and shivering. Psychol. Behav. 6, 391–397. 166 W.-X. Pan, N. McNaughton / Progress in Neurobiology 74 (2004) 127–166 Wilson, A., Kapp, B.S., 1994. Effect of lesions of the ventrolateral periaqueductal gray on the Pavlovian conditioned heart rate response in the rabbit. Behav. Neural Biol. 62, 73–76. Wirtshafter, D., Stratford, T.R., Shim, I., 1998. Placement in a novel environment induces Fos-like immunoreactivity in supramammillary cells projecting to the hippocampus and midbrain. Brain Res. 789, 331– 334. Woodnorth, M.-A., McNaughton, N., 2002a. Benzodiazepine receptors in the medial-posterior hypothalamus mediate the reduction of hippocampal theta frequency by chlordiazepoxide. Brain Res. 954, 194–201. Woodnorth, M.-A., McNaughton, N., 2002b. Similar effects of medial supramammillary or systemic injections of chlordiazepoxide on both theta frequency and fixed-interval responding. Cognit. Affect. Behav. Neurosci. 2, 76–83. Woodruff, M.L., Baisden, R.H., Whittington, D.L., Benson, A.E., 1987. Embryonic hippocampal grafts ameliorate the deficit in DRL acquisition produced by hippocampectomy. Brain Res. 408, 97–117. Wyss, J.M., Swanson, L.W., Cowan, W.M., 1979. A study of subcortical afferents to the hippocampal formation of the rat. Neuroscience 4, 463–476.









































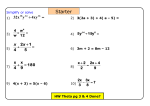
![Theorem [On Solving Certain Recurrence Relations]](http://s1.studyres.com/store/data/007280551_1-3bb8d8030868e68365c06eee5c5aa8c8-150x150.png)




