* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Plant Cell Vacuoles
Survey
Document related concepts
G protein–coupled receptor wikipedia , lookup
Extracellular matrix wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
SNARE (protein) wikipedia , lookup
Protein moonlighting wikipedia , lookup
Cytokinesis wikipedia , lookup
Cell membrane wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Magnesium transporter wikipedia , lookup
Cytoplasmic streaming wikipedia , lookup
Western blot wikipedia , lookup
Signal transduction wikipedia , lookup
Proteolysis wikipedia , lookup
Transcript
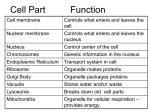
Plant Cell Vacuoles Secondary article Article Contents Jean-Marc Neuhaus, University of Neuchâtel, Neuchâtel, Switzerland Enrico Martinoia, University of Neuchâtel, Neuchâtel, Switzerland . Introduction . Vacuolar Constituents Most plant cells contain one or several vacuoles, which may occupy up to 95% of the cellular space. The vacuoles often appear empty under a light microscope (hence their name), except when they contain pigments or precipitated substances. The vacuole is delimited from the cytosol by the vacuolar membrane, which is also called tonoplast. Introduction The vacuole is the largest compartment of a mature plant cell and may occupy up to 95% of the total cell volume. In such mature cells, the cytosol is visible only as a thin layer, which is separated from the cell wall by the plasma membrane, and from the vacuolar sap (cell sap) by the vacuolar membrane (tonoplast). The constituents of the cell sap are mainly inorganic salts and water. The vacuole, therefore, enables the plant to reach a large size and a large surface area by accumulating salts from the environment, which drive further water uptake osmotically. Thus only a minimum of energy is diverted from the energy-consuming synthesis of metabolites. The requirement for cytosolic ions to be in homeostasis is also met by the vacuole as it serves as an internal reservoir of metabolites and nutrients (Matile, 1978; Boller and Wiemken, 1986; Martinoia, 1992). It can also be used for storage of other compounds that would be problematic in the cytosol, such as ions, pigments, storage compounds or toxic substances. Vacuolar Constituents Vacuolar constituents may vary between and within plant species, depending on the environmental conditions. Primary metabolites such as carbohydrates, amino acids and organic acids, as well as inorganic ions such as chloride, nitrate, potassium and sodium, are among the solutes usually present in the cell sap. Most of these solutes are stored only temporarily in the vacuole. In contrast, many compounds of secondary plant metabolism are thought to be sequestered definitively within the vacuole. Since many of these compounds are toxic and often play a role in plant defence, detoxification of the cytosol and storage of weapons against predators can be regarded as further functions of the vacuole. However, recent results indicate that even these compounds may be at least partially recycled. A third class of substances found within the vacuole are man-made chemicals (xenobiotics) that have been modified by the plant to cope with these potentially toxic compounds. . Plant Cells Can Possess More Than One Type of Vacuole . Vacuole Biogenesis . Transport Processes across the Vacuolar Membrane In most cases, the enzymatic activities found in the vacuole are restricted to hydrolases, which are mainly localized in this compartment (Matile, 1978; Boller and Wiemken, 1986). It has therefore been suggested that the vacuole corresponds to the lysosomes of animal cells. Additionally, several transferases are present in the vacuole. The best investigated so far are involved in fructan synthesis. Specialized vacuoles (protein bodies) from grains accumulate storage proteins as nitrogen source for the seedlings as well as phytate as phosphate store. Plant Cells Can Possess More Than One Type of Vacuole Plant biochemists have been puzzled by the large number of compounds found in vacuoles, some of which seemed incompatible. This can be explained by the existence of different vacuoles with different functions, such as lytic or storage compartments. In certain specialized cells different types of vacuoles can be distinguished optically because only one of the vacuoles contains a pigment or a precipitated substance, as in the cells of Mimosa leaves. Sometimes the difference is only visible in the electron microscope (Figure 1). It has recently become possible to label two different vacuolar compartments of single cells with antibodies recognizing two different tonoplast intrinsic proteins (TIP) or two different soluble vacuolar proteins (Paris et al., 1996). In many cells however, proteins of different types of vacuoles can also be found in the same large vacuole. This could be due to fusion of different types of vacuoles. Vacuole Biogenesis Origin of vacuoles Vacuoles are part of the secretory system of plants, and are ultimately derived from the endoplasmic reticulum (ER), as are the nuclear envelope, the Golgi apparatus and the ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net 1 Plant Cell Vacuoles Transport of soluble proteins to the vacuoles Soluble proteins are transported to preexisting vacuoles via the Golgi apparatus, as indicated by the typical modifications of glycans. Vesicles containing vacuolar proteins have also been visualized in the process of budding from the Golgi apparatus. Vacuolar proteins harbour positive sorting information that diverts them from the default pathway for soluble secretory proteins, which would otherwise result in secretion into the extracellular space. Vacuolar sorting signals Figure 1 Electron microscopic view of apple leaf cells possessing two different types of vacuoles (V1 containing a network of thick strands and a bright fringe, and V2). The other labelled structures are cell wall (CW), plasma membrane (PM), nucleus (N) with nucleolus (Nu), nuclear envelope (NE) with pores (Po), multivesicular body (MB) and dictyosomes (D). This figure was reprinted from Michel M, Gnägi H and Müller M (1992) Diamonds are a cryosectioner’s best friend. Journal of Microscopy 166: 43– 56, with permission of the Royal Microscopical Society. plasma membrane. Protein storage bodies of various cereals can be seen by electron microscopy to bud directly from the ER as storage proteins aggregate (Galili et al., 1993). In legumes, storage vacuoles are formed near preexisting lytic vacuoles, deriving apparently from a tubular-cisternal membrane system. In some meristematic cells, lytic vacuoles also seem to form by fusion and enlargement of ER or ER-derived tubules. Vacuoles can fuse to a single huge central vacuole during tissue differentiation, but they are also able to form smaller vacuoles by fission or blebbing. Fusion of different types of vacuoles can also occur, mixing storage and digestive proteins. 2 Analysis of several vacuolar proteins indicates that they are often synthesized as larger precursors with N- or Cterminal propeptides. Truncation of these propeptides results in secretion. Addition of the propeptides to different secreted proteins results in their vacuolar localization. These propeptides thus contain vacuolar sorting signals (VSS). Other vacuolar proteins appear to contain internal VSS. Comparison of sequences revealed that N-terminal propeptides of aleurain and sporamin contained a conserved motif NPIR (one-letter code for amino acids), and mutation analysis indicated that the I was the most important determinant, but the neighbouring positions were also involved, as not every substitution was tolerated. This sequence-specific VSS (ssVSS) is not necessarily located in an N-terminal propeptide but also functions when added to the C terminus of a reporter protein. This means that an ssVSS might also be located within a surface loop of a vacuolar protein, where it would be much more difficult to identify. Proteins with ssVSS appear to be targeted to lytic vacuoles. A putative vacuolar sorting receptor (VSR) for this first type of VSS was purified and cloned. Binding of the VSR was pH-dependent and showed a similar sequence specificity as found by the mutation analysis. Using VSR-binding as a test, a further ssVSS was tentatively identified in the C-terminal region of the 2S albumin from Brazil nuts. Analysis of peptides derived from the pumpkin 2S albumin identified two motifs, one of which contained a motif NLPS that is conserved in this protein family and also found in the N-terminal propeptide of several proteins related to sporamin. A second type of VSS was found in C-terminal propeptides of cereal lectins and of chitinases. Mutation analysis indicated little sequence specificity for these Cterminal VSS (ctVSS), except for the accessibility from the C-terminus. The terminus could be blocked by the addition of an N-glycosylation site or by glycines. Comparison of many C-terminal propeptides indicates the preference for a long side-chain at the terminal position and for a negative charge in one of the preceding positions. No sorting receptor has yet been identified for these ctVSS. Wortmannin, an inhibitor of phosphatidylinositol kinases, ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net Plant Cell Vacuoles γ α γ PVC α α SDV 2 γ γ PV γ δ δ δ δ δ Golgi Delta vacuole δ Default ER 3 δ γ ? γ LV CCV Transport of tonoplast proteins to the vacuoles Much less is known about the pathway of tonoplast proteins from the ER to the vacuole. The most abundant tonoplast proteins are the tonoplast intrinsic proteins (TIPs or aquaporins). Since different TIPs are localized in different vacuolar compartments, they have to be sorted by different mechanisms. The C-terminal transmembrane segment of a-TIP was sufficient to target a reporter protein to a tonoplast, with or without its cytosolic tail. Thus either this segment contains a tonoplast sorting signal or a tonoplast (which type of vacuole?) is the default destination for membrane proteins, as suggested for yeast. The Cterminal cytosolic tail of a-TIP actually prevented movement of a reporter protein from the ER to the Golgi, but α α α α 1 γ 2 γ α PSV δ Electron microscopic analysis has detected the formation of two different types of vesicles containing vacuolar proteins from the Golgi: clathrin-coated vesicles (CCV) and smooth dense vesicles (SDV). Clathrin-coated vesicles are well known from animal and yeast cells to participate in receptor-mediated transport, either from the trans-Golgi or from the cell surface to the endosomal compartment, an intermediate on the way to the lysosomes. The putative VSR for ssVSS was indeed isolated from pea CCVs. Smooth dense vesicles of pea cotyledons, however, were shown by immunocytochemistry to contain storage proteins in an aggregated form. The transport vehicle for vacuolar proteins with a ctVSS is unknown. Both transport pathways appear to involve prevacuolar compartments. Smooth dense vesicles carry storage proteins to multivesicular bodies, a kind of vacuole with numerous internal vesicles similar to endosomes of animal cells. For the lytic pathway, antibodies against the VSR detected both the Golgi and prevacuoles (approximately 250 nm) clustered near large vacuoles. These prevacuoles are likely to be the acidified compartment where the VSR releases its ligand and from which it recycles back to the Golgi. α Vesicles transporting proteins to vacuoles not to a vacuole. Finally, vacuolar sorting of soluble and membrane proteins was found to be differentially sensitive to inhibitors (monensin, brefeldin A), a further indication of different sorting pathways. The abundance of TIPs in tonoplasts beyond the presumable need for aquaporins (30–50% of total tonoplast proteins) even suggests a structural role. TIPs could be involved in the biogenesis of new vacuoles from the ER and could later function as identity markers for each type of vacuole. The plant cell could then prevent or allow the fusion of vacuoles of a given type with each other or with vacuoles of a different type and also with Golgiderived protein-containing vesicles. This model reconciles the vacuole biogenesis directly from the ER with the traffic of soluble proteins via the Golgi (Figure 2). α caused secretion of this type of proteins, at a concentration with no effect on the ssVSS-harbouring proteins, at least in tobacco BY-2 cells. Proteins with ctVSS appear to be targeted to nonlytic or storage vacuoles. The third type of VSS is protein-structure-dependent (psVSS) and has been found in several seed proteins. These tend to aggregate, once modifications in the Golgi apparatus have made them more hydrophobic. Aggregation is a possible nonreceptor-mediated sorting mechanism known to occur in specialized animal cells (secretory granules). Aggregation occurring in the ER seems to cause the formation of protein bodies in cereals. The effect of wortmannin on this transport system is unknown. δ CW Figure 2 Vacuole biogenesis in plants. Compartments of the secretory pathway are indicated: endoplasmic reticulum (ER); Golgi; cell wall (CW); and three types of vacuoles: protein storage vacuole (PSV, a-TIP, blue), lytic vacuole (LV, g-TIP, red) and ‘d vacuole’ (d-TIP, violet). The transport pathway from ER to Golgi involves concentration of proteins (grey spheres). Storage proteins (blue) aggregate in the Golgi near the rim of the cisternae and leave the Golgi in smooth dense vesicles (SDV) to a prevacuolar compartment (PVC) that fuses with the PSV. Clathrin-coated vesicles carry soluble proteins (red) selected by a receptor protein to a prevacuole (PV) from which the receptor recycles to the Golgi, while the cargo proteins continue to the LV. The pathway to ‘d vacuoles’ is unknown. The proteins lacking specific sorting determinants (green) are transported by default to the plasma membrane. Possible additional pathways are indicated: (1) direct transport of a-TIP from ER to PSV; (2) fusion of PSV and LV; and (3) exchanges or fusion between LV and ‘d vacuole’. Picture drawn by John Rogers. ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net 3 Plant Cell Vacuoles Transport Processes across the Vacuolar Channels and carriers Membrane Plant vacuoles may exercise different roles, depending on Vacuolar proton pumps Transport processes across membranes depend in many cases on the proton motive force (DpH and DC) generated by pumps residing in these membranes (Figure 3). The tonoplast contains two different proton pumps, an ATPase and a PPase (Rea and Sanders, 1987). V-type ATPases (VATPases) are multimeric complexes present on the endomembrane system of eukaryotic cells (Sze et al., 1992). They show some homologies with the F-ATPases present on the plasma membrane of eubacteria and on the inner membranes of mitochondria and chloroplasts and also form a characteristic ‘head and stalk’ structure. The VATPases can be distinguished from other ATPases by their highly specific inhibition by some antibiotics such as bafilomycin. V-ATPases are also found on other plant endomembranes such as the Golgi apparatus or ER, but it is still uncertain whether they are functional. The tonoplast proton-pumping pyrophosphatase (H+-PPase) is found widely distributed in higher plants as well as in algae, liverworts, mosses and ferns. Homologous proteins have been found in some bacteria, but so far not in animals or fungi. The tonoplast H 1 -PPase is a monomeric enzyme of about 87 kDa which is strictly dependent on Mg2 1 and activated by K 1 (Rea and Poole, 1993). GS-X ATP Cl–/NO3–? Mal2– K+/Ca2+ ADP+Pi PPi K+ H+ Calmodulin Pi+Pi Ca2+ cADPR Ca2+ IP3 ∆pH, ∆Ψ ATP Ca2+ H+ Na+ H+ ADP+Pi H+ H 2O Amino acids Glucose Sucrose Figure 3 Vacuolar transport systems. Pumps (red), transporters (green) and channels (blue) of the vacuolar membrane. For malate it is unclear whether a transporter or a channel is involved. Some channels are regulated by calmodulin, cyclic ADP-ribose (cADPR) or inositol trisphosphate (IP3), as indicated. GS-X, glutathion-conjugated xenobiotics. The picture is not exhaustive; for more details see text and the literature cited. 4 the nutritional state of a plant or whether a tissue is part of a source organ such as a leaf, or of a storage organ like a tuber. Therefore transport mechanisms may differ for different types of vacuoles. A case where different vacuolar transport mechanisms are described in different plants is sucrose, which is accumulated within sugarbeet tuber vacuoles by an H 1 -antiport mechanism, while facilitated diffusion is observed in barley, tomato and in stalk tissue of sugar cane. In barley and other fructan-accumulating species, sucrose is readily metabolized to higher polymers within the vacuole and trapped in this compartment. In this case, the energy for carbohydrate accumulation derives from the glycosidic bond between glucose and fructose and is not due to the vacuolar proton pumps. Similar differences as for sucrose may exist for glucose. In contrast to the members of the fructan family, which are not transported through the tonoplast, some members of the raffinose family are taken up by an H 1 -antiport mechanism in plants accumulating these sugars. Amino acid concentrations are often lower in the vacuole than in the cytosol. A transporter modulated by free ATP catalyses the transfer of most amino acids. A similar transport system has been described for the exchange of peptides, polyamines, cations and inorganic anions. Since the free ATP level is low in the cytosol and therefore only a very low transport activity of this transport system can be observed under physiological conditions, it has been suggested that it may play a role either in cytosolic homeostasis or as an H 1 -shunt mechanism. Inorganic and organic anion uptake is driven by the DC. Malate and citrate are the main organic acids accumulated in large quantities within the vacuole. Inhibition experiments suggest that one carrier or channel is responsible for the uptake of most di- and tricarboxylates. Patch clamp studies indicate that a separate channel is involved in the export of organic acids. Furthermore, inorganic anions such as chloride and nitrate most probably use a different transport system. In the case of nitrate it must be postulated that an additional force is driving its vacuolar uptake, since the observed membrane potential difference would not be sufficient to drive the concentration difference observed between the cytosol and the vacuole. Chloride channels are apparently activated by a protein kinase in stomata vacuoles. Several cation-permeable channels have been observed in the tonoplast. The SV (slow vacuolar) channel opens mainly at cytosolic positive membrane potentials, is permeable for potassium and calcium, and is activated in the presence of Ca2 1 . The SV channels are inhibited by calmodulin antagonists, indicating the involvement of calmodulin. In contrast, the FV (fast vacuolar) channel has a higher open probability at cytosolic negative membrane ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net Plant Cell Vacuoles potentials and is activated by low cytosolic Ca2 1 concentrations. Additionally, in stomata cells a K 1 channel thought to have a role in facilitating vacuolar K 1 release is described. For different plants a Na 1 /H 1 exchange mechanism, which may play a crucial role in salt tolerance, has been demonstrated, while a K 1 /H 1 antiport also seems to exist in some cases but has not been described in detail. Three different calcium channels have been reported to reside on the vacuolar membrane: an IP3-dependent channel; a cyclic ADP-ribose (cADPR)-dependent channel; and a Ca2 1 channel (see Figure 3). The IP3-dependent channel allows the release of calcium and the cADPRdependent channel is also responsible for the release of Ca2 1 from the vacuole. For both the IP3- and the cADPRdependent Ca2 1 channels, however, the spectrum of physiological stimuli to which the vacuolar receptors respond has yet to be elucidated. The Ca 2 1 channel is activated by membrane hyperpolarization. This channel is potently inhibited by La3 1 and Gd3 1 . Vacuolar calcium uptake is mediated by a Ca2 1 /H 1 exchange mechanism and by a Ca2 1 -ATPase. The vacuole as detoxification compartment: the role of ABC transporters A classical function of the central vacuole is the role as storage compartment for potentially toxic metabolites, such as phenolics or alkaloids, which may serve as repellents for herbivores or which are toxic for microorganisms. Beside such plant-generated substances, potentially toxic chemicals or pesticides (xenobiotics) can be modified by the plant and stored within the vacuole. An efficient detoxification requires transport mechanisms that strongly accumulate the potentially toxic compound within the vacuole. In several cases, as for coumarylglucosides or flavonoids, it has been shown that proton antiport mechanisms drive the vacuolar accumulation. Furthermore, trapping mechanisms such as protonation or conformational changes have been observed to be involved in vacuolar accumulation of secondary compounds. Metabolism of xenobiotics to glucosides or to glutathione conjugates is usually considered as a detoxification process, but these products may exert other biological activities. Removal of such conjugates from the cytosol could thus represent a crucial process in xenobiotic detoxification. Vacuolar transport of glutathione conjugates is not energized by the vacuolar proton gradient but directly by ATP. The uptake is inhibited by vanadate and other nucleotides can partially substitute for ATP. Very similar kinetics have been described for animal glutathione conjugate transporters. On the molecular level, it has been shown that several genes can mediate glutathione conjugate transport in plants. These transporters belong to the ABC (ATP-binding cassette) family and are highly homologous to glutathione conjugate transporters of fungi and animals (Rea et al., 1998). It is interesting to note that in animals glutathione conjugates are transported at the plasma membrane and are excreted in the surrounding medium. In contrast to the plant-generated glucosides, pesticides conjugated to glucose may also be transported by a directly energized transporter, probably also of the ABC type. Direct energization by ATP allows the creation of a much higher gradient between the cytosol and the vacuole and it is therefore supposed that the plant uses this type of transporter where the potential toxicity of a product is high. The degradation products of chlorophyll, open-chain tetrapyrroles that are still able to absorb light, accumulate within the vacuole by the same transporters responsible for glutathione conjugate transport. Upon illumination, these degradation products produce very dangerous radicals that would be lethal in a metabolically active compartment. Aquaporins Owing to large size, the central vacuole of plants plays an important role as water reserve and hence in water stress. Water fluxes across biological membranes were long thought to occur only through the lipid bilayer. However, it was recently shown that in many cases special proteins, known as aquaporins, mediate the exchange of water between the extracellular medium and the cell as well as within the cell. For vacuoles different types of aquaporins have been described, the best known being the a- and the gTIPs (tonoplast intrinsic proteins). g-TIPs can be found mainly in the large central vacuole and are strongly expressed in young, expanding tissue as well as in vacuoles of moving cells like Mimosa pulvini. a-TIPs are preferentially present in storage vacuoles where they are probably involved in dehydration. Interestingly, water permeability of a-TIPs, but not g-TIPs, is induced by phosphorylation. References Boller T and Wiemken A (1986) Dynamics of vacuolar compartmentation. Annual Review of Plant Physiology 37: 137–164. Galili G, Altschuler Y and Levanony H (1993) Assembly and transport of seed storage proteins. Trends in Cell Biology 3: 437–442. Martinoia E (1992) Transport of solutes in vacuoles of higher plants. Botanica Acta 105: 232–245. Matile P (1978) Biochemistry and function of vacuoles. Annual Review of Plant Physiology 29: 193–213. Paris N, Stanley CM, Jones RL and Rogers JC (1996) Plant cells contain two functionally distinct vacuolar compartments. Cell 85: 563–572. Rea PA, Li Z-S, Lu YP, Drozdowicz YM and Martinoia E (1998) From vacuolar GS-X pumps to multispecific ABC transporters. Annual Review of Plant Physiology and Plant Molecular Biology 49: 727–760. Rea PA and Poole RJ (1993) Vacuolar H 1 -translocating pyrophosphatase. Annual Review of Plant Physiology and Plant Molecular Biology 44: 157–180. Rea P and Sanders D (1987) Tonoplast energization: two H 1 pumps, one membrane. Physiologia Plantarum 71: 131–141. ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net 5 Plant Cell Vacuoles Sze H, Ward JM and Lai S (1992) Vacuolar H 1 -translocating ATPases from plants: structure, function and isoforms. Journal of Bioenergetics and Biomembranes 24: 371–381. Further Reading Leigh RA and Sanders D (1997) The plant vacuole. In: Callow JA (ed.) Advances in Botanical Research, vol.25. San Diego, CA: Academic Press 6 Kreuz K, Tommasini R and Martinoia E (1996) Old enzymes for a new job – herbicide detoxification in plants. Plant Physiology 111: 349– 353. Maurel C (1997) Aquaporins and water permeability of plant membranes. Annual Review of Plant Physiology and Plant Molecular Biology 48: 399–429. Neuhaus J-M and Rogers JC (1998) Sorting of proteins to vacuoles in plant cells. Plant Molecular Biology 38: 127–144. Robinson DG and Hinz G (1997) Vacuole biogenesis and protein transport to the plant vacuole: a comparison with the yeast vacuole and the mammalian lysosome. Protoplasma 197: 1–25. ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net