* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Cortical and subcortical afferents to the nucleus reticularis tegmenti
Biology of depression wikipedia , lookup
Metastability in the brain wikipedia , lookup
Visual selective attention in dementia wikipedia , lookup
Affective neuroscience wikipedia , lookup
Optogenetics wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Emotional lateralization wikipedia , lookup
Time perception wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Neuroeconomics wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Human brain wikipedia , lookup
Cortical cooling wikipedia , lookup
Neuroesthetics wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Aging brain wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Orbitofrontal cortex wikipedia , lookup
Hypothalamus wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Synaptic gating wikipedia , lookup
Basal ganglia wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Neuroplasticity wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Inferior temporal gyrus wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
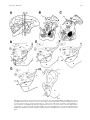
Visual Neuroscience (2001), 18, 725–740. Printed in the USA. Copyright © 2001 Cambridge University Press 0952-5238001 $12.50 DOI: 10.1017.S0952523801185068 Cortical and subcortical afferents to the nucleus reticularis tegmenti pontis and basal pontine nuclei in the macaque monkey ROLAND A. GIOLLI,1 KENNETH M. GREGORY,2 DAVID A. SUZUKI,3 ROBERT H. I. BLANKS,1 FAUSTA LUI,4 and KATHLEEN F. BETELAK 3 1 Department Department 3 Department 4 Department 2 of Anatomy and Neurobiology, College of Medicine, University of California, Irvine of Biological Sciences, California State University, Long Beach of Ophthalmology, Indiana University School of Medicine of Biomedical Sciences, University of Modena and Reggio Emilia, Via Campi 287, 41100 Modena, Italia (Received February 6, 2001; Accepted July 25, 2001) Abstract Anatomical findings are presented that identify cortical and subcortical sources of afferents to the nucleus reticularis tegmenti pontis (NRTP) and basal pontine nuclei. Projections from the middle temporal visual area (MT), medial superior temporal visual area (MST), lateral intraparietal area (LIP), and areas 7a and 7b to the basal pontine nuclei were studied using 3 H-leucine autoradiography. The results complemented a parallel study of retrograde neuronal labeling attributable to injecting WGA-HRP into NRTP and neighboring pontine nuclei. Small 3 H-leucine injections confined to MT, MST, LIP, area 7a, or area 7b, produced multiple patches of pontine terminal label distributed as follows: (1) An injection within MT produced terminal label limited to the dorsolateral and lateral pontine nuclei. (2) Injections restricted to MST or LIP showed patches of terminal label in the dorsal, dorsolateral, lateral, and peduncular pontine nuclei. (3) Area 7a targets the dorsal, dorsolateral, lateral, peduncular, and ventral pontine nuclei, whereas area 7b projects, additionally, to the dorsomedial and paramedian pontine nuclei. Notably, no projections were seen to NRTP from any of these cortical areas. In contrast, injections made by other investigators into cortical areas anterior to the central sulcus revealed cerebrocortical afferents to NRTP, in addition to nuclei of the basal pontine gray. With our pontine WGA-HRP injections, retrograde neuronal labeling was observed over a large extent of the frontal cortex continuing onto the medial surface which included the lining of the cingulate sulcus and cingulate gyrus. Significant subcortical sources for afferents to the NRTP and basal pontine nuclei were the zona incerta, ventral mesencephalic tegmentum, dorsomedial hypothalamic area, rostral interstitial nucleus of the medial longitudinal fasciculus, red nucleus, and subthalamic nucleus. The combined anterograde and retrograde labeling data indicated that visuo-motor cortico-pontine pathways arising from parietal cortices target only the basal pontine gray, whereas the NRTP, together with select pontine nuclei, is a recipient of afferents from frontal cortical areas. The present findings implicate the existence of parallel direct and indirect cortico-pontine pathways from frontal motor-related cortices to NRTP and neighboring pontine nuclei. Keywords: Corticopontine projections, Visual cortical areas, Frontal cortical areas, Parietal cortical areas, Nucleus reticularis tegmenti pontis, Basal pontine nuclei, Autoradiography, WGA-HRP, Anterograde & retrograde neuronal labeling agents into the cortex (i.e. cortico-centric studies) and cerebellocentric studies where lesions or anatomic tracers were placed within regions of cerebellum. The possibility that tracers injected into the internuncial regions of the pons would yield further insights and a more detailed and complete anatomic view has not been thoroughly pursued. Basal pontine afferents from visual cortical areas, including primary visual cortex (area 17), areas 18 and 19, superior temporal cortex, and posterior parietal cortex, have been studied in nonhuman primates employing a variety of tract-tracing techniques (Spatz & Tigges, 1973; Künzle & Akert, 1977; Brodal, 1978; Introduction A major proportion of the primate motor repertoire is comprised of visually guided behaviors. The neural substrate underlying some of these behaviors includes parallel cortico-ponto-cerebellar pathways which have been deduced primarily from neuroanatomical tract-tracing studies following injections of anterograde transport Address correspondence and reprint requests to: Roland A. Giolli, Department of Anatomy and Neurobiology, College of Medicine, University of California, Irvine, CA 92697-1275, USA. E-mail: [email protected] 725 726 Wiesendanger et al., 1979; Glickstein et al., 1980; Galletti et al., 1982; Maunsell & Van Essen, 1983a,b; Leichnetz et al., 1984; Leichnetz, 1989; Ungerleider et al., 1984; Weber & Yin, 1984; Huerta et al., 1986; May & Andersen, 1986; Stanton et al., 1988; Schmahmann & Pandya, 1989, 1991, 1993; Fries, 1990; Boussaoud et al., 1992; Baizer et al., 1993). The cortico-pontine projections from these cortical areas terminate in a variety of nuclei in the basal pontine gray. The nucleus reticularis tegmenti pontis (NRTP) appears to be excluded from the collection of termination sites (May & Andersen, 1986; present results). Cortical projections that do include terminations in NRTP arise from frontal areas such as the frontal (FEF) and supplementary (SEF) eye fields as well as the premotor and motor cortices (e.g. Shook et al., 1990). Within basal pontine nuclei, the frontal cortical projections overlap inputs from the parietal cortical areas. Given the growing body of knowledge concerning the functions of certain pontine structures receiving cortical inputs, the design of future physiological studies would benefit greatly from a better understanding of the anatomic details. What are needed are injections of tract tracers into the pons to show possible cortical and subcortical afferent sources that previously have not been apparent in “top–down”, cortico-centric anatomical investigations as enumerated above. In the macaque, Glickstein et al. (1985) were the first to address this need with relatively massive injections of HRP that incorporated large portions of the pons. The intent of the present study was to extend these studies with injections of WGAHRP that involved fewer pontine structures. By utilizing a “bottom– up” approach, we could compare the results of WGA-HRP injections into NRTP and neighboring pontine gray with the findings obtained with radioactive tracer injected into cerebral cortical areas. Brief reports of these findings have been presented (Giolli et al., 1995; Suzuki et al., 1998). Methods Autoradiography The projections of cerebrocortical areas onto basal pontine nuclei were analyzed in six adult monkeys (Macaca mulatta) after injections of 3 H-leucine and light-field autoradiography. Different cytoarchitecturally defined cerebral cortical areas were injected individually (viz., areas 17, PGa, OAa, POa, and 7a, 7b). All surgeries were performed in an AAALAC accredited surgical facility under aseptic conditions. Each monkey was preanesthetized with ketamine (10 mg0kg) plus acepromazine (0.5 mg0kg) and anesthetized through inhalation of 1.5–2.0% isofluorane. A portion of calvarium was removed with a dental drill and the dura mater transected carefully to expose the cerebral cortex. The monkeys were placed on a heating pad in order to maintain normal body temperature. The isotope, 3 H-leucine, was dissolved in physiological saline to a concentration of 33 mCi0ml. Two or three closely spaced injections (0.5 ml each, 0.5-mm separation) were made with finely drawn glass microelectrodes (Blanks et al., 1982). Injections were placed on the right and left sides of the cortex to optimally utilize these animals. Thus, bilateral 3 H-leucine injections were produced in cerebral cortical areas given the entirely ipsilateral or uncrossed nature of the corticopontine projections (e.g. Spatz et al., 1970; Spatz & Tigges, 1973; Tigges et al., 1973; Brodal, 1978; Wiesendanger et al., 1979; Maunsell & Van Essen, 1983a; Leichnetz et al., 1984; May & Andersen, 1986; Fries, 1990; Leichnetz, 1990). Each side was considered as a separate case and designated R.A. Giolli et al. Abbreviations Aq ai as AV Br.p Br. SC ce CG cg ca CL CM CP D dBC DL DM DMH ec FEF H1-H2 INC IC ip la L LG LH LIP LP lu mc MD MG MIP ML MLF MN MST MT NOT NRTP, Rt n.3 OAa oi p P Ped PGa PM Po, POa PP Pul.i Pul.l Pul.m Pul.o R RN riMLF R Rt, NRTP SEF Sg.Li, SNc SNr cerebral aqueduct arcuate sulcus, inferior branch arcuate sulcus, superior branch anterior ventral nucleus, thalamus brachium pontis brachium, superior colliculus central sulcus cingulate gyrus cingulate sulcus calcarine fissure nucleus centralis lateralis nucleus centromedian cerebral peduncle dorsal pontine gray decussation, brachium conjuntivum dorsolateral pontine gray dorsomedial pontine gray dorsomedial hypothalamic area external calcarine fissure frontal eye field H1 and H2 fields interstitial nucleus, Cajal internal capsule intraparietal sulcus lateral fissure lateral pontine gray lateral geniculate nucleus, pars dorsalis lateral hypothalamus lateral intraparietal area nucleus lateralis posterior lunate sulcus pars magnocellularis, medial geniculate nucleus nucleus medialis dorsalis medial geniculate nucleus medial intraparietal area medial lemniscus medial longitudinal fasciculus mammillary nuclear complex medial superior temporal visual area middle temporal visual area nucleus of the optic tract nucleus reticularis tegmenti pontis nucleus, oculomotor nerve cytoarchitectonic area OAa inferior occipital sulcus principal sulcus peduncular pontine gray peduncle, corticobulbar & corticospinal tracts cytoarchitectonic area PGa paramedian pontine gray posterior nucleus, thalamus cytoarchitectonic area POa posterior pretectal nucleus pulvinar, inferior division pulvinar, lateral division pulvinar, medial division pulvinar, oral division thalamic reticular nucleus red nucleus rostral interstitial nucleus, medial longitudinal fasciculus thalamic reticular nucleus nucleus reticularis tegmenti pontis supplemental eye field nucleus suprageniculatus0nucleus limitans substantia nigra, pars compacta substantia nigra, pars reticulata Afferents to basal pons St sts V VA, VIP VL, VMT VP VPI VPL VPLc VPM ZI 3N 4 6 7a, 7b 9 44 47 III subthalamic nucleus superior temporal sulcus ventral pontine gray ventral anterior nucleus, thalamus ventral intraparietal area central lateral nucleus, thalamus ventral mesencephalic tegmentum nucleus ventralis posterior nucleus ventralis posterior intermedialis nucleus ventralis posterior lateralis nucleus centralis lateralis, pars caudalis nucleus ventralis posterior medialis zona incerta oculomotor nerve area 4 area 6 areas 7a, 7b area 9 area 44 area 47 third ventricle 727 Wheat-germ agglutinin-horseradish peroxidase (WGA-HRP) The goal was to make an injection in NRTP. The location of NRTP was determined from stereotaxic coordinates taking into account the functionally and histologically determined locations in six previous monkeys. In two monkeys, one Macaca nemestrina (NEM1) and one Macaca fascicularis (FAS1), 0.1 ml (0.1 mg0ml) of WGA-HRP (Sigma L7017, St. Louis, MO) was injected with a Hamilton syringe over a 10-min period. The injection needles were left in place for more than 15 min before being slowly removed. Perfusions were performed 48 h postinjection using a sequence of isotonic saline, buffered paraformaldehyde plus glutaraldehyde, and buffered 10%, 20%, and 30% sucrose. After removal, each brain was placed in phosphate-buffered 30% sucrose for 24–36 h. All 50-mm sections were reacted with tetramethylbenzidine (Mesulam, 1978) and subsequently counterstained with neutral red. The experimental and surgical protocols employed for the autoradiography and WGA-HRP studies complied with the policies of the United States Public Health Service concerning the care and use of laboratory animals. Results Injection sites accordingly as M-1R, M-IL, M-2R, and so forth. All injections were placed stereotaxically in relationship to the topography of the visual cortical areas (Hoffmann et al., 1991), aided by direct stereomicroscopic examination of relevant gyri, sulci, and vascular landmarks of the cerebrum. The locations of the injection sites were subsequently confirmed using certain cytoarchitectural features and the patterns of corticothalamic projections from each area studied (see Results). Each delivery of tracer lasted 10–15 min, followed by a 10-min period prior to the removal of the micropipette. Postinjection, the dura mater and skin were sutured. Each monkey received an intramuscular injection of Ampicillin (12 mg0kg) to prevent infection and an intramuscular injection of Torbutrol (Butophanol tartrate) (0.1 ml of a 0.5 mg0ml solution IM) every 6 h for pain. Monkeys were then transferred to a recovery room and monitored continuously until they had recovered completely, that is, until the animals were ambulatory. After postoperative survival periods of 5–7 days, animals were again deeply anesthetized with Ketamine0Isofluorane and perfused transcardially with physiological saline followed by 10% formalin. Brains were blocked stereotaxically in the coronal plane and removed. Tissue blocks were embedded in paraffin, serially sectioned at 15 mm, and every fourth section mounted onto histologic slides. Mounted sections were coated in Kodak NTB-2 nuclear track emulsion, exposed in the dark at 48 C for 6–10 weeks, developed in Kodak D-19, fixed with Kodak Polimax T fixer, counterstained with thionin and coverslipped using the protocol of Cowan et al. (1972). The sections were examined under bright- and dark-field illumination. Microprojector drawings were made of sections through the injection site of each case to show the extent of the 3 H-leucine injections in relation to cortical cytoarchitecture. Microprojector drawings were also made of every other mounted section through the pons in order to study the organization of fields of terminal label in the basal pons. Drawings of the cortico-thalamic projections were used to provide validation for the location of the cortical injections. Autoradiography Labeled tracer was injected into several cortical areas. In two cases (M-1R and M-2R, not shown), injections were limited to area 17 located dorsomedial to the external calcarine fissure, and caudal to the lunate sulcus, that is, within area 17 representing central vision (Talbot & Marshall, 1941, their Fig. 5; Myers, 1965; Zeki, 1969, see his Fig. 10). The column of terminal label in the dorsal lateral geniculate nucleus in each case was restricted to the central region of the caudal half of this nucleus (Brouwer & Zeeman, 1926; Malpeli & Baker, 1975). However, neither of these area 17 cases showed terminal axonal labeling in the NRTP and basal pontine complex. The remaining anterograde cases produced terminal labeling in the basal pons and these are described below. Injections were made in MT and MST in superior temporal cortex (Fig. 1). The 3 H-leucine injection in case M-7L was restricted to area MT as shown in Figs. 1A and 1B, cytoarchitecturally equivalent to area OAa of Seltzer and Pandya (1978, 1989). This injection was small and limited to the caudal region of MT. It involved primarily the infragranular laminae with extension into the underlying white matter. The injection in M-8R was restricted to the caudal region of MST (Figs. 1D and 1E), corresponding cytoarchitecturally to area PGa of Seltzer and Pandya (1978, 1989). Cases M-8L and M-9R (Fig. 2) involve injections restricted to the lateral convexity of the inferior parietal lobule, originally designated cytoarchitecturally as area 7 (subdivided into 7a and 7b by Vogt & Vogt, 1919) and subsequently so defined by Weber and Yin (1984) and Andersen et al. (1990). Areas 7a and 7b correspond, respectively, to areas PG and PGF of von Bonin and Bailey (1947), Pandya and Seltzer (1982), and Schmahmann and Pandya (1989, 1991, 1993). The injection site in M-8L (Figs. 2A and 2B) lies primarily in area 7a with minimal extension rostrally into area 7b. The injection in M-9R (Figs. 2D and 2E) is almost entirely within the cytoarchitectural area 7b with minimal involvement of adjoining area 7a. 3 H-leucine was also injected into LIP (Figs. 4A and 4B), which is equivalent to the caudal part of area POa as 728 R.A. Giolli et al. Fig. 1. Corticopontine fiber projections were charted after 3 H-leucine injections into caudal areas of MT (A–C) and MST (D–F). A: The MT injection site (crosshatch) is reflected onto the surface of the cerebrum from the depth of the superior temporal sulcus in case M-7L. B: The MT injection site is shown in coronal sections with the core in black and the flare in stipple. C: The patches of terminal axonal label within basal pontine gray were limited to dorsolateral (DL) and lateral (L) pontine gray. D: The MST injection site, located in the superior temporal sulcus, is projected onto the cerebral surface in case M-8R. E: The MST injection site is depicted in coronal sections with the core and flare indicated in black and stipple, respectively. F: Patches of terminal label were observed in dorsal (D), dorsolateral (DL), lateral (L), and peduncular (P) pontine nuclei. The patches of label form a C-shaped lamella that is open ventromedially. The box in section 445 denotes the pontine region corresponding to the photomicrograph shown in Figs. 3A and 3B. Afferents to basal pons Fig. 2. Corticopontine projections arising from areas 7a (A–C) and 7b (D–F). A: The 3 H-leucine injection was in area 7a and is illustrated in black on the cerebral surface for case M-8L. There was minimal involvement of caudal area 7b. B: The core (black) and flare (stipple) are indicated. C: Patches of terminal axonal label were distributed over a widespread region in the basal pontine gray, which includes the dorsal (D), dorsolateral (DL), lateral (L), peduncular (P), and ventral (V) pontine nuclei. The patches of terminal label form a prominent C-shaped lamella that is open medially. The box in section 455 denotes the pontine region corresponding to the photomicrograph shown in Figs. 3C and 3D. D: Indicated in black is an injection centered predominantly in area 7b with slight impingement on rostral area 7a in case M-9R). E: The injection site is seen in coronal sections with the core in black and flare in stipple. F: Clusters of terminal axonal label were distributed over a large field of the basal pontine gray that included all sectors of the pontine gray. Thus, the dorsomedial (DM) and paramedian (PM) pontine zones were added to the list of nuclei receiving inputs from area 7a (see panels A–C). These patches were aligned in a circular lamella. The box in section 449 indicates the pontine zone corresponding to the photomicrograph shown in Figs. 3E and 3F. 729 730 cytoarchitecturally defined by Pandya and Seltzer (1982) and Seltzer and Pandya (1986). WGA-HRP The injection site for WGA-HRP in NEM1 (Figs. 7B and 7C) included NRTP, dorsomedial pontine nucleus (DM), dorsal pontine nucleus (D), and portions of peduncular pontine nucleus (P) and probably the paramedian pontine nucleus (PM). The nomenclature of Nyby and Jansen (1951) was used to label regions of the basal pontine gray, acknowledging that it is difficult to definitively establish nuclear boundaries based upon neuronal size and shape, neuronal packing density, intensity of Nissl staining, or pattern of fascicles of the basis peduncularis. The results of our autoradiographic study are consistent with the exclusion of dorsolateral (DL), lateral (L), and ventral pontine nucleus (V) from the HRP uptake zone. Confirmation of injection sites The locations of the cortical areas injected in MT, MST, 7a, 7b, and LIP were determined as closely as possible through examination of the cytoarchitecture of cortical areas. The cytoarchitecture was difficult to make out within the core of the injections. However, in the flare regions of injections, and surrounding the injections (i.e. outside the core region), the cytoarchitecture was discernible. Yet, at times, it was difficult to see where one cortical area ended and an adjacent area began. In addition, there were often wide transitional zones present between cortical areas. Thus, we feel that the cortical boundaries depicted in broken lines in Figs. 1, 2, 4, 6, and 8 present approximations for the boundaries of the cortical areas injected with 3 H-leucine. To better establish the location of tracer injections in functional areas of cortical areas, we have relied heavily upon chartings made of the corticothalamic projections (Figs. 5A–5E), which we compared with equivalent chartings and descriptions from other studies in which the cortical projections from areas MT, MST, 7a, 7b, and LIP to the dorsal thalamus of monkeys had been studied using neuronal transport techniques alone or combined with electrophysiology. Our results thus show that (1) with an injection into caudal area MT (Figs. 1A and 1B), we found terminal fields in the medial, lateral, and inferior division of pulvinar (as defined by Olszewski, 1952) and the midcaudal one-third of the thalamic reticular nucleus (Fig. 5A) with a total pattern quite consistent with anterograde transport studies performed by Maunsell and Van Essen (1983a, their Fig. 12), and Ungerleider et al. (1984), in which electrophysiological identification of area MT had been performed prior to tracer injections. (2) Our injection into caudal area MST (Figs. 1D and 1E) produced patches of terminal label in the medial, lateral, and inferior pulvinar and, ventrally, in the thalamic reticular nucleus (Fig. 5B), with the total pattern matching the descriptions of Boussaoud et al. [1992, their Fig. (3)], who identified MST electrophysiologically before making their injections of neuronal tracer. 3) After an injection involving chiefly area 7a (Figs. 2A and 2B), we observed terminal axonal fields in the medial division of the pulvinar and dorsal part of the thalamic reticular nucleus (Fig. 5C), with an organization that closely resembles that reported by Weber and Yin (1984) following an area 7a injection. (4) In addition, after an injection centered largely in area 7b (Figs. 2D and 2E), a pattern of terminal fields is seen within the medial and oral divisions of pulvinar and the dorsal-to-central part of the thalamic reticular nucleus (Fig. 5D) that clearly matches descriptions of corticothalamic projections from area 7b by Weber and Yin (1984) and R.A. Giolli et al. Asanuma et al. (1985). (5) After an 3 H-leucine injection into caudal area LIP (Figs. 4A and 4B), terminal fields were found in the medial, lateral, and inferior divisions of pulvinar and the dorsal segment of the thalamic reticular nucleus (Fig. 5E) exhibiting a pattern paralleling the descriptions by Asanuma et al. (1985) and Baizer et al. (1993) based on tracer injections into area LIP. Termination sites Autoradiography In all cases, 3 H-leucine injections into parietal cortical areas resulted in terminal axonal labeling in different sets of ipsilateral pontine nuclei. By contrast, pontine terminations were not observed with 3 H-leucine injections within primary visual cortex (cases M-1R and M-2R). Notably, in none of these cases was there evidence of labeled axon terminals in NRTP. Thus, NRTP was excluded as a terminus for these cortical neurons located in parietal injection sites. On the other hand, each of these cases produced characteristic multiple patches of cortico-pontine terminal labeling within the basilar pontine nuclei. The individual patches usually ranged in size from 40 mm to 60 mm in diameter up to 200– 300 mm in diameter. The MT injection resulted in circumscribed pontine axonal labeling in DL and dorsal portions of L (Fig. 1C). Labeled axon terminals resulting from the MST injection were further identified in pontine nuclei D and P, in addition to DL and L (Fig. 1F). Injection into area 7a produced labeled terminals in D, DL, L, P, and V (Fig. 2C), whereas injection into area 7b resulted in terminal labeling in these same pontine nuclei with the addition of DM and PM (Fig. 2F). Fig. 3 shows the arrangements of patches of terminal labeling within the pontine nuclei in three of the anterogradely injected cases. After a discrete 3 H-leucine injection into LIP, patches of terminal labeling were found in DL, L, P, and D (see Fig. 4C). WGA-HRP The retrograde neuronal labeling of cortical neurons following injections of WGA-HRP into the basal pons was pertinent to the anterograde studies described above. Thus, all of the corticopontine projectional data were confirmed by the retrograde studies, except those from area MT (case M-7L) in which we believe that the pontine nuclei receiving MT projection were not included in the WGA-HRP injection sites in either NEM1 or FAS1. Specifically, retrograde labeling was observed in sections of cortex that included MST, 7a, 7b, LIP, VIP, as well as the medial intraparietal area, referred to as MIP by Colby et al. (1988), as located in the medial bank of the intraparietal sulcus opposite to LIP (Fig. 8D). Labeled cells were also observed in cortex lining the caudal segment of the cingulate sulcus located on the medial surface of the parietal cortex. No retrograde labeling was seen in MT which, based upon the autoradiography findings described above, excluded DL and L from the WGA-HRP uptake zones in NEMI1 and FAS1. Contrasted with the pontine projections from parietal cortical areas, projections of frontal areas have been shown by others to include axonal terminations in NRTP (e.g. Shook et al., 1990). With our WGA-HRP injections in NRTP and adjacent pontine gray (Figs. 7B and 7C), retrograde neuronal labeling was found over a broad area of frontal cortex extending well beyond the boundaries of the frontal (FEF) and supplementary (SEF) eye fields (Figs. 8A– 8C). The retrograde neuronal labeling extended throughout much Afferents to basal pons Fig. 3. Matching bright- and dark-field photomicrographs show examples of the patches of terminal axonal label seen in the basal pontine nuclei. Arrows in corresponding bright and dark photomicrographs point to equivalent structures as observed with bright- and dark-field illuminations. Several of the patches of terminal labeling are not seen to good advantage in the bright-field photomicrographs depicted in Figs. C and E, but the locations of these patches, as observed in the dark-field photomicrographs, has been determined and shown in the corresponding bright-field pictures. A, B: (MST, case M-8R) The field is boxed in Fig. 1F, section 445 and includes portions of dorsal, dorsolateral, lateral, and peduncular pontine gray. In this case, compared with the others depicted in these photomicrographs, the size and shape of patches varied considerably and were of high density. C, D: (area 7a, case M-8L) This field is enclosed by the box in Fig. 2C, section 455. E, F: (area 7b, case M-9R) The rectangular area viewed is indicated in Fig. 2F, section 449. The borders of the sections in C and E have been drawn in with pen. Calibration bars in B, D, and F ⫽ 500 mm. 731 732 R.A. Giolli et al. Fig. 4. Corticopontine fiber projections have been mapped after an injection into caudal area LIP in case M-4R. A: The injection site in caudal LIP (crosshatch) is transferred onto the cerebral surface. B: Coronal sections reveal the core (black) and flare (stipple) of the injection. C: The distribution and density of the patches of terminal label occupied portions of the dorsal (D), dorsolateral (DL), lateral (L), and peduncular (P) pontine gray. The patches are seen to form a crude C-shaped lamella. of the medial surfaces of frontal cortex including the cingulate sulcus and the cingulate gyrus. Retrogradely labeled somata were observed in a variety of subcortical nuclei and regions as demonstrated in Figs. 7A, 7D–7H following the WGA-HRP injection into the pons (Figs. 7B and 7C). Densely labeled regions include portions of the zona incerta (Figs. 7D–7F) and dorsomedial hypothalamic area adjacent to the third ventricle (Fig. 7H). Moderately dense labeling was charted in the ventral mesencephalic tegmentum dorsal to the substantia nigra (Figs. 7F and 7G). Additionally, light but consistent neuronal labeling was found within the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF) (Figs. 7D–7G), red nucleus (Fig. 7D), and subthalamic nucleus (Figs. 7D, 7F, and 7G). Discussion The results of this study advance our understanding of the afferents to some of the pontine nuclei that are involved in the processing of sensori-motor signals. The most novel findings were the subcortical sources of inputs into the NRTP and0or adjacent pontine nuclei, WGA-HRP uptake zone. These sources were the zona incerta, riMLF, ventral mesencephalic tegmentum, red nucleus, subthalamic nucleus, and dorsomedial hypothalamic area. In addition, the results provide more refined support for the existence of projections to the macaque NRTP and0or adjacent pontine nuclei from MIP (named by Colby et al., 1988), the cingulate cortex as located in the depths of the cingulate sulcus and onto the cingulate gyrus, premotor cortex on the medial part of the cerebrum, and a significant area of frontal cortex. These cortical sources of pontine projections are in addition to previously known projections from extensive areas of the parietal and frontal cortices. While definitive conclusions cannot be reached on the basis of the one or two injections per area in this study, the results, combined with those of others, support the notion that many cortical and subcortical motor- and oculomotor-related structures send afferents to NRTP and0or adjacent pontine nuclei. With respect to the NRTP, these results are consistent with the evolving view of a global role for NRTP in motor control and complement physiological observations that implicate the NRTP in eye and head movement control (Crandall & Keller 1985; Gamlin & Clarke 1995; Suzuki et al., 1997, 1999, 2001; Yamada et al., 1996). The autoradiographic and retrograde tracer results are complementary and facilitate a comparison of “top–down” (i.e. anterograde tracers placed in cortex) versus “bottom–up” (i.e. retrograde tracers placed in NRTP0pons) approaches to studying afferents to pontine structures. The autoradiographic results indicated that many of the visuo-motor-related parietal areas project to pontine nuclei DL and L. In addition, MST and LIP also project to D and P (Figs. 1F and 4C, respectively). Areas 7a and 7b project to all of the above pontine nuclei and, additionally, target the ventral pontine nucleus (see Figs. 2C and 2F). Area 7b had the most widespread projection pattern of the cortical areas studied; its cortical projections included all of the nuclei targeted by 7a, and it was the sole source of afferents to DM and PM in the present study Afferents to basal pons Fig. 5. Chartings show the corticothalamic projections for each of the cortical injections illustrated in Figs. 1, 2, and 4. A–E indicate the corticothalamic chartings, in order, for (A) Figs. 1A–1B, (B) Figs. 1D–1E, (C) Figs. 2A–2B, (D) Figs. 2D–2E, and (E) Figs. 4A– 4B. Case descriptions are given on the left, and numbers of the individual coronal sections of brain are provided with each section. Figs. 1A–1E have appeared as parts of Figs. 3–7 of Lui et al. (1995). 733 734 Fig. 6. Semidiagrammatic representation of the cerebral cortical injections of 3 H-leucine into functional areas of cerebral cortex in relationship to the distribution of labeled pyramidal cells resulting from injection of WGAHRP into the NRTP0pontine nuclei as illustrated for cases NEMI1 and FAS1 in Figs. 6B and 6C. Note the presence of retrogradely labeled cortical neurons within zones for anterogradely injected 3 H-leucine in areas LIP, 7a, 7b, and MST as seen in a coronal section through the posterior parietal cortex. The lack of retrograde neuronal labeling in area MT confirms that the dorsolateral pontine nucleus was not in the WGA-HRP uptake zone. (Fig. 2F, sections 418, 430, & 441). These corticopontine projections are essentially similar to those reported by other authors studying nonhuman primates (Spatz & Tigges, 1973; Brodal, 1978; Wiesendanger et al., 1979; Galletti et al., 1982; Maunsell & Van Essen, 1983a; Ungerleider et al., 1984; Weber & Yin, 1984; May & Andersen 1986; Schmahmann & Pandya, 1989, 1991, 1993; Fries, 1990; Boussaoud et al., 1992; Baizer et al., 1993). Some differences include the reports of Ungerleider et al. (1984) and Maunsell and Van Essen (1983a) of additional MT projections to peduncular pontine gray (P), and also Schmahmann and Pandya (1989), Weber and Yin (1984), and Wiesendanger et al. (1979) findings of a light area 7b projection to the DM with no projection to the PM. With respect to the latter, our results are consistent with those of May and Andersen (1986) in showing a more substantive area 7b projection to DM and a light projection to PM. These differences could be due to slightly differing determinations of pontine nuclei borders or slight differences between injection sites and amount of tracer injected. The autoradiographic data were important in characterizing the extent of the WGA-HRP uptake zones of the retrograde studies (i.e. the NRTP0basal pontine uptake zones). The lack of retrograde labeling in MT, following pontine WGA-HRP injections, was interpreted as indicating that DL and L were excluded from the uptake zone. The retrograde labeling in area 7b was presumably due to tracer uptake in DM. Whether PM was also included in the uptake zone could not be ruled out though the core of the WGAHRP injection site appears to be just dorsal to the edge of PM R.A. Giolli et al. (Figs. 7B and 7C). Although NRTP was included in the WGAHRP injection site, our autoradiographic results indicate that the injected parietal vision- and visuo-motor-related cortical areas do not project to NRTP. Similar results were implicit in the absence of any statement that labeled cells were observed in NRTP in the autoradiographic studies of others. Only May and Andersen (1986) stated explicitly that area 7a, 7b, dorsal prelunate, and LIP do not project to NRTP. Thus, we concluded that retrograde labeling of cells in cortical areas MST, 7a, 7b, and LIP was due to uptake into DM, D, and0or dorsal portion of P. Leichnetz et al. (1984) and Glickstein et al. (1985) were, to our knowledge, the earliest and only investigators to utilize a bottom–up approach to identifying primate cortico-pontine pathways. Glickstein et al. sought to identify all the cortical areas projecting to the pons and intentionally employed large HRP-injection volumes designed to incorporate the entire pontine gray and NRTP on one or both sides of the pons. Interestingly, our much more restricted NRTP0pontine uptake zone resulted in a distribution of retrograde labeling that was similar to that of Glickstein et al. (1985) with the most notable difference being the lack of labeling of MT (due to the exclusion of DL and L from our uptake zone). In Cebus, a New World monkey, Leichnetz et al. (1984) placed HRP gel in NRTP and observed retrograde labeling over much of frontal cortex and in both banks of the intraparietal sulcus, LIP, and MIP. This latter observation could reflect a species difference between capuchine and macaque monkeys, but the labeling in LIP could suggest spread of the HRP into pontine nucleus D making it uncertain if the MIP labeling in Cebus was due to fiber projections to NRTP or D or both nuclei. The projections from cingulate sulcus to the pons have been revealed only with the bottom–up approach used in this study and by Glickstein et al. (1985). To be sure, Vilensky and Van Hoesen (1981) conducted an autoradiographic study of cingulate-pontine projections, but their tracer injection sites were limited to cingulate gyrus and did not appear to invade the depths of the cingulate sulcus. Thus, our observation of retrograde labeling in the depths of the cingulate sulcus refine those of Glickstein et al. (1985) by revealing projections to a constrained region of the pontine gray near NRTP from cingulate cortex lying in the depths of the cingulate sulcus and extending onto the cingulate gyrus. In contrast to findings concerning parieto-pontine projections, the subcortical projections of frontal cortical areas include NRTP as a terminus. In the macaque monkey, frontal and supplementary eye fields (FEF and SEF, respectively) have been shown to project to NRTP as well as to neighboring pontine nuclei (Künzel & Akert, 1977; Brodal, 1980; Huerta et al., 1986; Stanton et al., 1988; Shook et al., 1990). In these studies, the use of quite circumscribed tracer injections or lesions did not give any indication of the extent of frontal cortex projecting to NRTP and basilar pontine gray. The retrograde neuronal labeling seen in Figs. 8A–8C, together with the section indicators in Fig. 8 (upper right insert), allows for an appreciation of the large amount of frontal cortex targeting the macaque NRTP0pontine region. These results are similar to those of Glickstein et al. (1985) obtained with massive HRP uptake sites and of Leichnetz et al. (1984) for the Cebus monkey. The sources of cortical projections to the NRTP0pontine region included much of the frontal cortex extending onto the medial wall and continuing into the cingulate sulcus and over the cingulate cortex (Figs. 8A– 8C). The Cebus monkey appears to be similar to the macaque, as comparable results were obtained in Cebus monkeys with HRP-gel implants in NRTP (Leichnetz et al., 1984). The results of our combined autoradiographic and retrograde studies for cortical Afferents to basal pons Fig. 7. Retrograde labeling of subcortical neurons was charted following an injection of WGA-HRP into the NRTP0pontine region in case NEM1. A: The locations of the planes of section for the chartings as depicted in B–H. B,C: Coronal sections through the injection site with section B rostral to C and the approximate plane of sections for B–C indicated by an asterisk in A. D–H: Mappings of subcortical afferents to the NRTP0pontine nuclei. Retrogradely labeled neurons were present in section D: red nucleus (RN); D–F: zona incerta (ZI); F, G: ventral mesencephalic tegmentum (VMT); D–F: rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF); D–G: subthalamic nucleus (St); and H: dorsomedial hypothalamic area (DMH). Each dot represents one retrogradely labeled neuron. 735 736 R.A. Giolli et al. Fig. 8. Cerebrocortical neuronal labeling is seen following the injection of WGA-HRP into the NRTP and neighboring pontine gray in case NEM1 (see Figs. 6B– 6C for injection site). The plane of section for A–D is shown on lateral and medial views of the surface of cerebrum at upper right. Chartings A–D are in rostro-caudal sequence, and these gauge the density and distribution of retrogradely labeled pyramidal cells after the injection into the NRTP0pontine area. Each dot equals one labeled cortical neuron. projections to the NRTP0pontine region are summarized in Fig. 9A. Area MT was not included as retrograde labeling was not observed in area MT, and the anterograde results indicate an MT projection to pontine nuclei DL and L, which are not depicted in Fig. 9A. The retrograde axonal labeling that we found in subcortical nuclei raises the possibility of parallel, direct, and indirect path- ways from frontal cortical areas to the NRTP0basal pons. As indicated by the broken lines in Fig. 9B, axonal projections have been demonstrated from FEF to the superior colliculus, red nucleus, riMLF, zona incerta, and subthalamic nucleus (Künzle & Akert 1977; Huerta et al., 1986; Stanton et al., 1988; Shook et al., 1990). As we observed retrograde neuronal labeling in these Afferents to basal pons 737 Fig. 9. The afferents to the NRTP0pontine region are shown in “neuronal flow diagrams” A and B. As per the reasons given in the text, it is probable that the WGA-HRP uptake zone in cases NEM1 and FAS1 included NRTP, the dorsomedial (DMPN), dorsal (DPN), and sectors of the peduncular (PPN) pontine gray. A: Summarizes the cortical sources of afferents to the NRTP0pontine nuclei. The projections that are either novel or refinements in the macaque are shown in bold type, whereas previously known sources of cortico-pontine fibers are indicated in regular-size type. B: Provides summaries of the subcortical sources of inputs to the NRTP0pontine nuclei. Findings from the present research are depicted in bold type with previously observed sources of input shown in regular type. Thicker arrows denote more substantial inputs to the pontine complex than do thinner arrows. Dashed lines indicate cortical inputs targeting subcortical pontine afferents. Note the potential for indirect cortico-subcortical-NRTP0pontine region pathways that parallel direct pathways demonstrated in A. The dotted lines around DPN and PPN attest to the common pontine terminal axonal zones for projections from LIP, VIP, 7a, 7b, and MST. structures (with the exception of the superior colliculus which was not examined), indirect pathways from FEF to NRTP via these structures may parallel the direct FEF-NRTP pathways noted above. Similarly, FEF and SEF projections to superior colliculus (Shook et al., 1990) may be part of a FEF0SEF-SC-NRTP path that parallels the direct FEF0SEF-NRTP pathway uncovered by Shook et al. (1990) and demonstrated in the present study (Figs. 7 and 8). Furthermore, it is known that motor cortex (M1) projects directly upon NRTP and, also, the red nucleus (Hartmann-von Monakow et al., 1979, 1981; Leichnetz, 1986), therefore, the M1-NRTP direct path parallels an M1-red nucleus-NRTP pathway. These possibilities are intriguing, though the existence of parallel direct and indirect pathways requires that the cortical inputs to red nucleus, riMLF, zona incerta, subthalamic nucleus, and superior colliculus terminate on exactly those neurons that project onto NRTP. Whether the cortical inputs to these subcortical structures do make these requisite connections remains to be determined. The present findings, combined with the potential indirect pathways, 738 are summarized in Fig. 9B, in which notable data from the present study are indicated in bold lettering. Functional considerations Much is known about the visual, oculomotor, and0or motor properties of cells in most of the parietal areas wherein retrograde labeling was observed and will not be reviewed here (for reviews see Andersen et al., 1997 and Colby & Goldberg, 1999). The finding of retrograde labeling in the MIP, the cingulate cortex (lining the cingulate sulcus and continuing inferiorly onto the cingulate gyrus and superiorly onto the superior parietal lobule), and a myriad of areas in the frontal lobe raises questions about the functions that may be mediated by the cortico-pontine fibers arising in these areas. Somatosensory, visual, and reaching responses have been recorded in MIP cells (Colby & Duhamel, 1991; Johnson et al., 1996). MIP receives inputs from visual area PO and projects to frontal, motor-related cortices (Colby et al., 1988; Matelli et al., 1998). Motor-related activity has been recorded in portions of the medial extensions of supplementary and presupplementary motor areas referred to as the “cingulate motor area” (Shima & Tanji, 1998) and the “medial wall” (Picard & Strick, 1996). Recording sites for motor-related activity extended into the cingulate sulcus, but not more ventrally. The functions of retrogradely labeled frontal areas exclusive of FEF, SEF, premotor, and motor cortices remain to be determined. Focusing attention on the neural substrate for the smoothpursuit eye movement system, two parallel cortico-ponto-cerebellar pathways have been implicated: one involving neurons in the pontine nuclei proper and the other incorporating the NRTP. Our autoradiographic results are consistent with an MT0MST-pontine nucleus DL-cerebellum pathway that bypasses the NRTP. Visual motion signals of utility for guiding pursuit and pursuit-related modulations in activity have been recorded in structures of the MT0MST-pontine nucleus DL-cerebellum (e.g. Miles & Fuller, 1975; Lisberger & Fuchs, 1978; Maunsell & Van Essen, 1983b; Komatsu & Wurtz, 1988; Mustari et al., 1988; Newsome et al., 1988; Suzuki & Keller, 1988a,b; Thier et al., 1988; Suzuki et al., 1990), yet bilateral lesions of DL did not abolish pursuit behavior (May & Keller, 1988) suggesting a parallel pathway involved with pursuit. Our WGA-HRP results are consistent with an FEF-NRTPcerebellum route. Activity related to smooth-pursuit eye movements have been noted in these structures (Miles & Fuller, 1975; Lisberger & Fuchs, 1978; Suzuki & Keller, 1988a,b; Gottlieb et al., 1994; Suzuki et al., 1999) adding support to the validity of this second cortico-ponto-cerebellar pathway as a component of the neural substrate regulating pursuit behavior. The functions of certain of the subcortical sources of inputs to the NRTP0basal pontine gray have not been completely characterized. However, the red nucleus and rostral interstitial nucleus of MLF are well known to be involved with motor and oculomotor functions, respectively (see, e.g. King & Fuchs, 1979; Gibson et al., 1985). Saccade-related pauses in activity have been recorded in zona incerta implicating its role in oculomotor control (Ma, 1996). The subthalamic nucleus, as part of the basal ganglia circuitry, may permit the NRTP0pontine nuclei to monitor the function of the basal ganglia. However, we are uncertain about the functional significance of the dorsomedial hypothalamic area and ventral mesencephalic tegmental with their afferents to the NRTP0 pontine nuclei. Though understanding awaits further investigations, the known functional attributes of subcortical structures R.A. Giolli et al. projecting to the NRTP0pontine nuclei are consistent with oculomotor and0or motor-related roles. Taken together, our results and the results of others implicate the existence of three parallel cortico-ponto-cerebellar systems, a parieto-ponto-cerebellar system, and two fronto-ponto-cerebellar systems. The massive HRP volumes that Glickstein et al. (1985) injected into the pontine gray including NRTP had the advantage of labeling all the cerebral cortical sources of pontine afferents. Large portions of the parietal and frontal cortices were retrogradely labeled. Our autoradiographic and WGA-HRP results provided a finer resolution of the view of cortico-pontine connectivity and, combined with the results of others, implicate a parieto-pontocerebellum and a fronto-ponto-cerebellum system of connections that exclude NRTP, and a fronto-NRTP-cerebellum set of connections. Whether the patches of pontine nuclei cells receiving inputs from parietal cortex are the same as those receiving inputs from frontal cortex remains to be determined. The challenge will be to clarify the functional differences between these three systems of cortico-NRTP0ponto-cerebellum connections. A hint may be available from the nature of the parallel MT0MST-pontine and FEF0 SEF-NRTP substrates for smooth-pursuit eye movement control discussed above. Without excluding significant motor-related parietal responses, one wonders if the sensory information in the sensori-motor, parieto-pontine pathways might distinguish these connections from more “motor” related fronto-NRTP0pontine connections. An added complication is the possibility that the three direct cortico-NRTP0ponto-cerebellum systems may be paralleled by indirect cortico-pontine pathways through subcortical structures to NRTP0pontine nuclei that were implicated in the current study. Acknowledgments We wish to gratefully acknowledge the expert technical contributions of Mr. Shag Van Pham. This research was supported by NSF Grant # IBN-9121376 (to R.A.G.), NIH Grant EY09082 (to D.A.S.), and part of an unrestricted departmental award from Research to Prevent Blindness to the Department of Ophthalmology, Indiana University School of Medicine (for K.F.B.). References Andersen, R.A., Asanuma, C., Essick, G. & Siegel, R.M. (1990). Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. Journal of Comparative Neurology 296, 65–113. Andersen, R.A., Snyder, L.H., Bradley, D.C. & Xing, X. (1997). Multimodal representation of spaces in the posterior parietal cortex and its use in planning movement. Annual Review of Neuroscience 20, 303–330. Asanuma, C., Andersen, R.A. & Cowan, W.M. (1985). The thalamic relations of the caudal inferior parietal lobule and the lateral prefrontal cortex in monkeys: Divergent cortical projections from cell clusters in the medial pulvinar nucleus. Journal of Comparative Neurology 241, 357–381. Baizer, J.S., Desimone, R. & Ungerleider, L.G. (1993). Comparison of subcortical connections of inferior temporal and posterior parietal cortex in monkeys. Visual Neuroscience 10, 59–72. Blanks, R.H.I., Giolli, R.A. & Pham, S.V. (1982). Projections of the medial terminal nucleus of the accessory optic system upon pretectal nuclei in the pigmented rat. Experimental Brain Research 48, 228–237. Boussaoud, D., Desimone, R. & Ungerleider, L.G. (1992). Subcortical connections of visual areas MST and FST in macaques. Visual Neuroscience 9, 291–302. Brodal, P. (1978). The corticopontine projection in the rhesus monkey. Origin and principles of organization. Brain 101, 251–283. Brodal, P. (1980). The cortical projection to the nucleus reticularis Afferents to basal pons tegmenti pontis in the rhesus monkey. Experimental Brain Research 38, 19–27. Brouwer, B. & Zeeman, W.P.C. (1926). The projection of the retina in the primary optic neuron in the monkey. Brain 49, 1–35. Colby, C.L. & Duhamel, J.R. (1991). Heterogeneity of extrastriate visual areas and multiple parietal areas in the macaque monkey. Neuropsychologia 29, 517–537. Colby, C.L., Gattas, R., Olson, C.R. & Gross, C.G. (1988). Topographical organization of cortical afferents to extrastriate visual area PO in the macaque: A dual tracer study. Journal of Comparative Neurology 269, 392– 413. Colby, C.L. & Goldberg, M.E. (1999). Space and attention in parietal cortex. Annual Review of Neuroscience 22, 319–349. Cowan, W.M., Gottlieb, D.I., Hendrickson, A.E., Price, J.L. & Woolsey, T.A. (1972). The autoradiographic demonstration of axonal connections in the central nervous system. Brain Research 37, 21–51. Crandall, W.F. & Keller, E.L. (1985). Visual and oculomotor signals in nucleus reticularis tegmenti pontis in alert monkey. Journal of Neurophysiology 54, 1326–1345. Fries, W. (1990). Pontine projections from striate and prestriate visual cortex in the macaque monkey. An anterograde study. Visual Neuroscience 4, 205–216. Galletti, C., Maioli, M.G., Squatrito, S. & Battaglini, P. P. (1982). Corticopontine projections from the visual area of the superior temporal sulcus in the macaque monkey. Arch Italian Biology 126, 411– 417. Gamlin, P.D.R. & Clarke, R.J. (1995). Single-unit activity in the primate nucleus reticularis tegmenti pontis related to vergence and ocular accommodation. Journal of Neurophysiology 73, 2115–2119. Gibson, A.R., Houk, J.C. & Kohlerman, N.J. (1985). Magnocellular red nucleus activity during different types of limb movement in the macaque monkey. Journal of Physiology 358, 527–549. Giolli, R.A., Gregory, K.M., Lui, F. & Blanks, R.H.I. (1995). Visuocortical projections to the macaque monkey basal pontine gray. Society for Neuroscience Abstracts 21, 656. Glickstein, M., Cohen, J.L., Dixon, B., Gibson, A., Hollins, M., Labossiere, E. & Robinson, F. (1980). Corticopontine visual projections in macaque monkeys. Journal of Comparative Neurology 190, 209–229. Glickstein, M., May, J.G., III. & Mercier, B.E. (1985). Corticopontine projections in the macaque: The distribution of labelled cortical cells after large injections of horseradish peroxidase in the pontine nuclei. Journal of Comparative Neurology 235, 343–359. Gottlieb, J.P., MacAvoy, M.G. & Bruce, C.J. (1994). Neural responses related to smooth-pursuit eye movements and their correspondence with electrically elicited smooth eye movements in the primate frontal eye field. Journal of Neurophysiology 72, 1634–1653. Hartmann-von Monakow, K., Akert, K. & Künzle, H. (1979). Projections of precentral and premotor cortex to the red nucleus and other midbrain areas in Macaca fascicularis. Experimental Brain Research 34, 91–105. Hartmann-von Monakow, K., Akert, K. & Künzle, H. (1981). Projection of precentral, premotor and prefrontal cortex to the basilar pontine grey and to nucleus reticularis tegmenti pontis in the monkey (Macaca fascicularis). Arch. Suisses Neurologie 129, 189–208. Hoffmann, K.-P., Distler, C. & Erickson, R. (1991). Functional projections from striate cortex and superior temporal sulcus to the nucleus of the optic tract (NOT) and dorsal terminal nucleus of the accessory optic system (DTN) of macaque monkeys. Journal of Comparative Neurology 313, 707–724. Huerta, M.F., Krubitzer, L.A. & Kaas, J.H. (1986). Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys, I. Subcortical connections. Journal of Comparative Neurology 253, 415– 439. Johnson, P.B., Ferraina, S., Bianchi, L. & Caminiti, R. (1996). Cortical networks for visual reaching: Physiological and anatomical organization of frontal and parietal lobe arm regions. Cerebral Cortex 6, 102–119. King, W.M. & Fuchs, A.F. (1979). Reticular control of vertical saccadic eye movements by mesencephalic burst neurons. Journal of Neurophysiology 42, 861–876. Komatsu, H. & Wurtz R.H. (1988). Relation of cortical areas MT and MST to pursuit eye movements. I. Localization and visual properties of neurons. Journal of Neurophysiology 60, 580– 603. Künzle, H. & Akert, K. (1977). Efferent connections of cortical area 8 739 (frontal eye field) in Macaca. A reinvestigation using the autoradiographic technique. Journal of Comparative Neurology 173, 147–164. Leichnetz, G.R. (1986). Afferent and efferent connections of the dorsolateral precentral gyrus (area 4, hand0arm region) in the Macaque monkey, with comparisons to area 8. Journal of Comparative Neurology 254, 460– 492. Leichnetz, G.R. (1989). Inferior frontal eye field projections to the pursuit-related dorsolateral pontine nucleus and middle temporal area (MT) in the monkey. Visual Neuroscience 3, 171–180. Leichnetz, G.E. (1990). Preoccipital cortex receives a differential input from the frontal eye field and project to the pretectal olivary nucleus and other visuomotor-related structures in the rhesus monkey. Visual Neuroscience 5, 123–134. Leichnetz, G.R., Smith, D.J. & Spencer, R.F. (1984). Cortical projections to the paramedian tegmental and basilar pons in the monkey. Journal of Comparative Neurology 228, 388– 408. Lisberger, S.G. & Fuchs, A.F. (1978) Role of primate flocculus during rapid behavioral modification of vestibulo-ocular reflex. I. Purkinje cell activity during visually guided horizontal smooth pursuit eye movement and passive head rotation. Journal of Neurophysiology 41, 733–763. Lui, F., Gregory, K.M., Blanks, R.H.I. & Giolli, R.A. (1995). Projections from visual areas of the cerebral cortex to pretectal nuclear complex, terminal accessory optic nuclei, and superior colliculus in macaque monkey. Journal of Comparative Neurology 363, 439– 460. Ma, T.P. (1996). Saccade-related omnivectoral pause neurons in the primate zona incerta. NeuroReport 7, 2713–2716. Malpeli, J.G. & Baker, F.H. (1975). The representation of the visual field in the lateral geniculate nucleus of Macaca mulatta. Journal of Comparative Neurology 161, 569–594. Matelli, M., Govoni, P., Galletti, C., Kutz, D.F. & Luppino, G. (1998). Superior area 6 afferents from the superior parietal lobule in the macaque monkey. Journal of Comparative Neurology 402, 327–352. Maunsell, J.H.R. & Van Essen, D.C. (1983a). The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. Journal of Neuroscience 3, 2563–2586. Maunsell, J.H.R. & Van Essen, D.C. (1983b). Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. Journal of Neurophysiology 49, 1127–1147. May, J.G., III & Andersen, R.A. (1986). Different patterns of corticopontine projections from separate cortical fields within the inferior parietal lobule and dorsal prelunate gyrus of the macaque. Experimental Brain Research 63, 265–278. May, J.G., III & Keller, E.L. (1988). Recovery from smooth pursuit impairments after successive unilateral and bilateral chemical lesions in the dorsolateral pontine nucleus of the monkey. In Post-Lesion Neural Plasticity, ed. Flohr, H., pp. 413– 420. Berlin: Springer-Verlag. Mesulam, M.M. (1978). A tetramethylbenzidine method for the light microscopic tracing of neural connections with horseradish peroxidase (HRP) neurohistochemistry. In Neuroanatomical Techniques. Short Course. Society for Neuroscience, pp. 65–71. Miles, F.A. & Fuller, J.H. (1975). Visual tracking and the primate flocculus. Science 189, 1000–1002. Mustari, M.J., Fuchs, A.F. & Wallman, J. (1988). Response properties of dorsolateral pontine units during smooth pursuit in the rhesus macaque. Journal of Neurophysiology 60, 664– 686. Myers, R.E. (1965). Organization of visual pathways. (A Discussion). In Functions of the Corpus Callosum, ed. Ettklinger, E.G., pp. 133– 138. London: Churchill. Newsome, W.T., Wurtz, R.H. & Komatsu, H. (1988). Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. Journal of Neurophysiology 6, 604– 620. Nyby, O. & Jansen, J. (1951). An experimental investigation of the corticopontine projection in Macaca mulatta. Skrifter utgitt av Det Norske Videnskaps-Akademi: Oslo1. Mat.-Naturv. Klasse. 3, 1– 47. Olszewski, J. (1952). The Thalamus of the Macaca mulatta. An Atlas for Use with the Stereotaxic Instrument. New York: S. Karger, 93 pp. Pandya, D.N. & Seltzer, B. (1982). Intrinsic connections & architectonics of posterior parietal cortex in the rhesus monkey. Journal of Comparative Neurology 204, 196–210. Picard, N. & Strick, P.L. (1996). Motor areas of the medial wall. A review of their location and functional activation. Cerebral Cortex 6, 342–353. 740 Schmahmann, J.D. & Pandya, D.N. (1989). Anatomical investigation of projections to the basis pontis from posterior association cortices in rhesus monkey. Journal of Comparative Neurology 289, 53–73. Schmahmann, J.D. & Pandya, D.N. (1991). Projections to the basis pontis from the superior temporal region in the rhesus monkey. Journal of Comparative Neurology 308, 224–248. Schmahmann, J.D. & Pandya, D.N. (1993). Prelunate, occipitotemporal, and parahippocampal projections to the basis pontis in rhesus monkey. Journal of Comparative Neurology 337, 94–112. Seltzer, B. & Pandya, D.N. (1978). Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Reseach 149, 1–24. Seltzer, B. & Pandya, D.N. (1986). Posterior parietal projections to the intraparietal sulcus of the rhesus monkey. Experimental Brain Research 62, 459– 469. Seltzer, B. & Pandya, D.N. (1989). Intrinsic connections and architectonics of the superior temporal sulcus in the rhesus monkey. Journal of Comparative Neurology 290, 451– 471. Shima, K. & Tanji, J. (1998). Role for cingulate motor area cells in voluntary movement selection based on reward. Science 282, 1335– 1338. Shook, B.L., Schlag-Rey, M. & Schlag, J. (1990). Primate supplementary eye field, I. Comparative aspects of mesencephalic and pontine connections. Journal of Comparative Neurology 301, 618– 642. Spatz, W.B., Tigges, J. & Tigges, M. (1970). Subcortical projections, cortical associations, and some intrinsic interlaminar connections of the striate cortex in the squirrel monkey (Saimiri ). Journal of Comparative Neurology 140, 155–174. Spatz, W.B. & Tigges, J. (1973). Studies on the visual area MT in primates. II. Projections to subcortical structures. Brain Research 61, 374–378. Stanton, G.B., Goldberg, M.E. & Bruce, C.J. (1988). Frontal eye field efferents in the macaque monkey. II. Topography of terminal fields in midbrain and pons. Journal of Comparative Neurology 271, 493–506. Suzuki, D.A., May, J.G., Keller, E.L. & Yee, R.D. (1990). Visual motion response properties of neurons in the dorsolateral pontine nucleus of the alert monkey. Journal of Neurophysiology 63: 37–59. Suzuki, D.A., Betelak, K.F. & Yee, R.D. (1997). Eye and head gazesaccade responses in monkey nucleus reticularis tegmenti pontis (NRTP). Society for Neuroscience Abstracts 23, 2367. Suzuki, D.A., Gregory, K.M., Giolli, R.A., Betelak, K.F. & Blanks, R.H.I. (1998). Cortical efferents to monkey dorsal pontine grey and0or nucleus reticularis tegmentis pontis (NRTP). Society for Neuroscience Abstracts 24, 145. Suzuki, D.A., Yamada, T., Hoedema, R. & Yee, R.D. (1999). Smoothpursuit eye-movement deficits with chemical lesions in macaque nu- R.A. Giolli et al. cleus reticularis tegmenti pontis. Journal of Neurophysiology 82, 1178–1186. Suzuki, D.A., Betelak, K.F. & Yee, R.D. (2001). Gaze pursuit responses in head-unrestrained macaque nucleus reticularis tegmenti pontis. Journal of Neurophysiology (pending revision). Suzuki, D.A. & Keller, E.L. (1988a). The role of the posterior vermis of monkey cerebellum in smooth-pursuit eye movement control. I. Eye and head movement-related activity. Journal of Neurophysiology 59, 1–18. Suzuki, D.A. & Keller, E.L. (1988b). The role of the posterior vermis of monkey cerebellum in smooth-pursuit eye movement control. II. Target velocity-related Purkinje cell activity. Journal of Neurophysiology 59, 19– 40. Talbot, S.A. & Marshall, W.H. (1941). Physiological studies on neural mechanisms of visual localization and discrimination. American Journal of Ophthalmology 24, 1255–1264. Thier, P., Koehler, W. & Buettner, U.W. (1988). Neuronal activity in the dorsolateral pontine nucleus of the alert monkey modulated by visual stimuli and eye movements. Experimental Brain Research 70, 496–512. Tigges, J., Tigges, M. & Kalaha, C.S. (1973). Efferent connections of area 17 in Galago. American Journal of Physical Anthropology 38, 393–398. Ungerleider, L.G., Desimone, R., Galkin, T.W. & Mishkin, M. (1984). Subcortical projections of area MT in the macaque. Journal of Comparative Neurology 223, 368–386. Vilensky, J.A. & Van Hoesen, G.W. (1981). Corticopontine projections from the cingulate cortex in the rhesus monkey. Brain Research 205, 391–395. Vogt, C. & Vogt. O. (1919). Ergebnisse unserer Hirnforschung. Vierte mitteilung: die physiologische bedeutung der architektonischen rindenreizungen. Journal of Psychology and Neurology 25, 279– 461. von Bonin, G. & Bailey, P. (1947). The Neocortex of Macaca mulatta. Urbana, Illinois: University of Illinois Press, 163 pp. Weber, J.T. & Yin, T.C.T. (1984). Subcortical projections of the inferior parietal cortex (area 7) in the stump-tailed monkey. Journal of Comparative Neurology 224, 206–230. Wiesendanger, R., Wiesendanger, M. & Ruegg, D.G. (1979). An anatomical investigation of the corticopontine projection in the primate (Macaca fascicularis and Siamiri sciureus)—II. The projection from frontal and parietal association areas. Neuroscience 4, 747–765. Yamada, T., Suzuki, D.A. & Yee, R.D. (1996). Smooth pursuit-like eye movements evoked by microstimulation in macaque nucleus reticularis tegmenti pontis. Journal of Neurophysiology 76, 3313–3324. Zeki, S.M. (1969). Representation of central visual field in prestriate cortex of monkey. Brain Reseach 14, 271–291.