* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 1 Carbonyl Condensation Reactions (Conjugate Addition) If we look
Aromaticity wikipedia , lookup
Marcus theory wikipedia , lookup
Elias James Corey wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Homoaromaticity wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Discodermolide wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Metal carbonyl wikipedia , lookup
Stille reaction wikipedia , lookup
Organosulfur compounds wikipedia , lookup
George S. Hammond wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Ene reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Petasis reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Hydroformylation wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
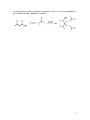
Car b on yl Co nd ensa tion Rea ct ions (C o nj ug ate Add ition ) If we look at resonance structures for conjugated carbonyl compounds (often called α,β-unsaturated compounds), we’ll see that there are TWO sites for nucleophilic attack. Thus, we’ll find that the alpha carbon is often quite nucleophilic and the β carbon is electrophilic. Addition of a nucleophile to the double bond to form an enolate, which tautomerizes to the ketone, is called conjugate addition. When carbonyl addition or conjugate addition will occur? In other words, when will a nucleophile add to the carbonyl, or the double bond of a conjugated carbonyl compound? We have seen that “hard” nucleophiles, such as present in RMgBr and RLi reagents, add to the carbonyl. A way to force only conjugate addition with an organometallic reagent is to form the copper salt - in this case, a dialkylcuprate. We’ve already seen these are used to prepare ketones from acid chlorides – formation of copper salts is a great way to “soften” a hard nucleophile. Thus, “soft” nucleophiles favor conjugate addition to α,β-unsaturated compounds. Hence non-carbon nucleophiles like nitrogen (amines) and oxygen (alcohols) will always add in a conjugate fashion, particularly if the reaction is carried out under neutral conditions. Some examples: Enolates are also “soft” nucleophiles and hence add to conjugated carbonyls in conjugate fashion. Ald ol Rea ct ion Two molecules of an aldehyde or ketone react with each other in the presence of base to form a β-hydroxy carbonyl compound: 1 O HO–, H2O O 2 H H + HO– HO O O O H H H O O H H OH In this reaction, an enolate is the nuclophile; one aldehyde molecule becomes the enolate while the other molecule serves as the electrophile. With aldehydes, the equilibrium usually favors the aldol products. But with ketones, the equilibrium usually favors starting materials. Of course, there are ways to derive the equilibrium towards products in either case. You should also note that an y carbonyl compound with alpha hydrogens can undergo the aldol reaction (i.e. esters, acids, etc.). How can we tell whether a molecule is going to undergo an aldol-like reaction, or an enolate-like reaction? There are a couple of clues: 1) STRONG base. If bases like LDA are being used, and a decent electrophile is present, then you’re likely looking at an e no late type reaction. 2) GOOD electrophiles. If there’s another strong electrophile around, such as a halogen molecule (Br2, for example), or something like iodomethane (CH3I), you’re looking at an en ola te type of reaction. 3) Catalytic amounts of a weak base. If t he r e are no ot he r e le ctr op hiles adde d, then the use of a “weaker” base, such as sodium ethoxide (NaOEt) or hydroxide (HO-) is usually a clue that an a ld o l-type reaction will be taking place. Ald ol C on den sat io n β-Hydroxy carbonyl compounds formed in the aldol reaction dehydrate more readily than other alcohols. In fact, under basic conditions, the β-hydroxy carbonyl compound is often not isolated; it loses elements of H2O from the α and β carbons to form an α,β-unsaturated compound. For example, 2 As you see, the ene-al is simply the aldol product minus the elements of water a condensation reaction. In fact, e lim inatio n o f wate r is sp on tan eo us a nd the β- h yd rox y car bo n yl co mp o un d is n o t is ola ted when the α,βunsaturated product is further conjugated with a C=C or a benzene ring: O O HO–, O H 2O Ph 2 Ph CH3 H3C HO HO– Ph -H2O Ph H3C Ph NOT ISOLATED So, one more time . . . the aldol condensation takes a carbonyl compound (usually an aldehyde, ketone or ester) and joins two of them together to form a conjugated carbonyl compound. Generally, with aldol condensations you are combining two of the same molecule to form a dimer. However, in a few cases it is possible to combine two different molecules to form the aldol product. There are two MAJOR restrictions: (1) One of the pair must not have any enolizable (i.e. alpha) protons (for example, aryl aldehydes & ketones like benzaldehyde or benzophenone). H OEt O OR (2) One of the pair must form an enol MUCH more easily than the other Cla ise n Co nd ensa tion But what about the enolates of other carbonyl compounds, such as esters? Do they undergo these “self-condensation” reactions as well? Of course they do! However, the thing to note is that esters have essentially a “leaving group” attached to the carbonyl carbon. Thus, the products of the condensation reaction are slightly different. In essence, the product of an aldol-type reaction between two esters is almost always a 1,3-dicarbonyl compound. In its simplest form: 3 (note - why do we use NaOEt, and not NaOH?). Again, this is a fairly general reaction – just about any ester can undergo this type of self-condensation. The “driving force” of this reaction is not dehydration, however – it is the formation of the stable enolate anion (i.e. the one between the two carbonyl groups) that makes the final step irreversible. Is it possible to do “mixed” Claisen reactions? Of course, provided the same rules are followed as for the mixed aldol. One component must either have no enolizable protons, or must be enolized VERY rapidly. Mechanism Practice!!! Draw the mechanism for the reaction above. M ichea l Add ition The nature of the electrophile is also worth considering. What if we have a conjugated carbonyl compound? Recall that we then add a stabilized nucleophile to the alkene portion – a 1,4 addition. Malonates and keto-esters are particularly good at this type of reaction, called a Michael addition: 4 If a large excess of the conjugated compound is used, it is of course possible to get a double-Michael (dialkylated) product: 5