* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download MOVEMENT OF SUBSTANCES ACROSS THE PLASMA MEMBRANE
Survey
Document related concepts
Model lipid bilayer wikipedia , lookup
Cytoplasmic streaming wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
Cell nucleus wikipedia , lookup
Membrane potential wikipedia , lookup
Cell growth wikipedia , lookup
Cell encapsulation wikipedia , lookup
Lipid bilayer wikipedia , lookup
Extracellular matrix wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cytokinesis wikipedia , lookup
Signal transduction wikipedia , lookup
Cell membrane wikipedia , lookup
Transcript
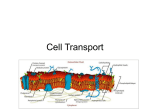
MOVEMENT OF SUBSTANCES ACROSS THE PLASMA MEMBRANE Substances Required by Cells and Substances Eliminated from Cells 1. Metabolism consists of two processes: a) Anabolism, which is the building up of molecules. Examples are the syntheses of proteins and ATPs, which a cell needs. b) Catabolism, which is the breaking down of large molecules to smaller, simpler molecules. An example is the oxidation of glucose in cell respiration. 2. A cell takes in raw substances for anabolism from external environment. 3. Wastes from catabolism and excess substances are removed from the cell. 4. Also, water and various salts are either brought into, or are taken out of the cell. Substances that leave the cell Substance Carbon dioxide (animal cell) Oxygen (plant cell) Secretions Nitrogenous waste Excess water Reason of Removal A waste product of respiration A waste product of photosynthesis Are cellular products for use in other parts of the body Are waste products from breakdown of excess proteins Osmoregulation Substances that enter the cell Substance Oxygen (animal cell) Carbon dioxide (plant cell) Glucose Amino acids Ionic salts Reason of intake For cell respiration As a raw material in photosynthesis For cell respiration As raw materials for protein synthesis For cell metabolism and osmoregulation The Necessity for Movement of Substances Across the Plasma Membrane (a) Cells need nutrients and oxygen. These pass through the plasma membrane from the external environment. (b) Cells produce waste products which exit through the plasma membrane. (c) The plasma membrane can control the types and the amounts of subtances needed by the cell at any time. The Structure of the Plasma Membrane 1. Figure below is an electron micrograph of two plasma membranes. 2. Each plasma membrane looks like a railway track. 3. Singer and Nicholson proposed the fluid mosaic model in 1972 to explain the structure of the plasma membrane. 4. The fluid mosaic model is the currently accepted model of the cell membrane. Epidermal cells (a) Epidermal cells, (b) Electron micrograph showing two plasma membranes of adjacent epidermal cells 5. To understand the concept of the plasma membrane, we shall look at the: (a) basic unit of the plasma membrane (b) formation of the plasma membrane (c) other molecules present in the plasma 'membrane 6. The basic unit of the plasma membrane is the phospholipid molecule. 7. The phospholipid molecule consists of: (a) a polar phosphate molecule head which is hydrophilic, and (b) two non-polar fatty acid tails which are hydrophobic 8. The meanings of the three technical words: (a) A polar molecule carries an unequal distribution of electric charges. This unequal distribution of electrical charges produces a polar molecule which can attract other polar molecules, such as water molecules. (b) Hydrophilic means "water-loving" or attracted to water molecules. (c) Hydrophobic means "water-hating," or repelling water molecules. 9. Formation of the plasma membrane. (a) Phospholipid units (polar heads) attract each other, grouping together side by side to form a layer of phospholipids (b) One layer of phospholipids forms over another to produce a phospholipid bilayer (c) In this phospholipid bilayer the: hydrophilic heads point outwards facing water molecules on both sides (th external environment, and the aquah cytoplasm). hydrophobic tails point inwards, away fron water molecules. (d) This phospholipid bilayer forms the frame work of the plasma membrane. 10. Other molecules present in the plasma membran are: (a) Cholesterol molecules which fit in between the phospholipids molecules, making the plasma membrane more rigid and stable. (b) Carrier proteins and channel protein which assist and control the movement of water-soluble ions and certain molecule across the membrane. (c) Glycolipids which are combinations of lipid and polysaccharides, help cells to recognise each other. (d) Glycoproteins which are combinations of proteins and polysaccharides, also hel cells to recognise each other. 11. Based on Figure below, the meaning of the "fIuid mosaic model” is clear because: (a) fIuid refers to the free movement of protein and phospholipids within the membrane, and (b) mosaic refers to the array of different protein embedded in the phospholipid bilayer. glycoprotein polysaccharide protein Permeability of the Plasma Membrane Permeability of the Phospholipid Bilayer 1. Permeable means "allowing something to pass through". 2. The proteins and the phospholipids of the plasma membrane affect the permeability of the plasma membrane. 3. The plasma membrane is selectively permeable or semi-permeable as it allows only certain substances to pass through it but not others. 4. The plasma membrane is only permeable to small molecules such as water, oxygen and carbon .dioxide. 5. The phospholipid bilayer is permeable to: a) small non-polar (hydrophobic) molecules that are lipid-soluble, such as: fatty acids, glycerol, steroids, vitamins A, D, E and K. b) small uncharged molecules, such as: water, oxygen and carbon dioxide. These molecules are small enough to squeeze through between the phospholipid gaps by simple diffusion or osmosis (for water) down their respective concentration gradients. 6. The phospholipid bilayer is not permeable to: a) large polar molecules that are not soluble in lipid, such as glucose, amino acids, nucleic acids and polysaccharides b) ions (charged), regardless of size, such as: H+, Na+, HC03-, K+, Ca2+, Cl-, and Mg2+. 7. To summarise, the phospholipid bilayer: a) is semi-permeable, and b) allows the entry of small uncharged or hydrophobic molecules, but c) prevents the entry of larger polar or even small charged substances. 8. The three characteristics of a molecule that determine its permeability across the plasma membrane are its: a) polarity - (hydrophobic as compared to hydrophilic) b) charge - (charged as compared to uncharged) c) size - (large as compared to small) Types of Transport Across the Plasma Membrane Solutes move across the plasma membrane by two main processes: (a) passive transport - which does not require a cell to use energy, and (b) active transport - which requires a cell to use energy to move molecules through its cell membrane 1)The Movement of Soluble Substances Across the Plasma Membrane Through the Process of Passive Transport A) Simple Diffusion 1. Simple diffusion is the random movement of ions or molecules from a region of their high concentration to a region of their low concentration down a concentration gradient until an equilibrium is achieved. 2. Molecules have kinetic energy, move randomly, and collide with each other. 3. There are more collisions in a region of high concentration than in a region of low concentration. 4. Random collisions of molecules spread the molecules out, down the concentration gradient (difference in concentration of a particular substance in one region compared to another region.) 5. Diffusion achieves a dynamic equilibrium when the concentration is the same in all regions. 6. The structure of the plasma membrane determines the types of molecules or ions that can cross it. 7. Molecules that can cross are (see Figure 3.4): (a) small non-polar (hydrophobic) molecules (b) small uncharged molecules 8. These molecules squeeze between the polar phospholipids heads, then dissolve in the lipid layers and come out on the other side. 9. Factors affecting the rate of diffusion: Factor Diffusion gradient Size of molecules /ions Temperature Diffusion medium Surface area Effect on the rate of simple diffusion The steeper the diffusion gradient, the higher the rate The smaller the size, the higher the rate The higher the temperature, the higher the rate Rate in gas>rate in liquid> rate in solid The larger the surface area, the higher the rate Examples of simple diffusion in biology: (i) Gaseous exchange in the alveoli: oxygen from air to blood; carbon dioxide from blood to air . (ii) Gaseous exchange in photosynthesis: carbon dioxide from air into the leaf; oxygen from leaf to air. (iii) Gaseous exchange in respiration: oxygen frOIL blood to tissue cells; carbon dioxide in the opposite direction. (iv) Osmosis: the diffusion of water through the semi-permeable membrane of the root hair cell . (v) The absorption of digested food through the villi of the small intestine. (iv) The absorption of oxygen by unicellular organisms. B) Facilitated Diffusion 1. Facilitated diffusion is the movement of specific molecules(or ions) across the plasma membrane 2. Facilitated diffusion is assisted either by pore proteins or by carrier proteins, and the direction of movement is down the concentration gradient of the molecules concerned. 3. No energy is required in facilitated diffusion 4. The functions of pore proteins and carrier proteins: a) Pore proteins (channel proteins) i. Charged ions (such as Na+, K+, Ca2+, and Cl-) cannot diffuse across the nonpole: centre of the phospholipids bilayer. ii. Pore proteins open up pores or channels across the membrane to allow entry or exit. iii. Each pore or channel is specific and will only allow one particular type of ion through. iv. Pores can open or close, acting as gates, to cater to the needs of the cells. b) Carrier protein i. They allow larger polar molecules (such as sugars and amino acids) to cross. ii. A particular protein attaches itself to the binding site of a carrier protein. iii Then, the carrier protein changes shape and delivers the molecule across the plasma membrane. C) Osmosis 1. Osmosis is the movement of water molecules from a region of low solute concentration (high water concentration) to a region of high solute concentration (low water concentration) through a semi-permeable membrane. 2. The semi-permeable membrane allows water molecules, but not the solute molecules to pass through. 3. Water continues to flow from the region of low solute concentration to the region of high solute concentration until the solute concentrations in both regions are the same 4. Examples of osmosis in biology a) Absorption of water by root hairs. b) Movement of water from one cell to another c) Absorption of water in the alimentary canal-stomach, small intestine and colon 2)The Movement of Soluble Substances Across the Plasma Membrane Through the Process of Active Transport 1. Active transport is the movement of substances across the plasma membrane from a region of low concentration to a region of high concentration. 2. In active transport, the substances move across a membrane against the concentration gradient. This transport requires work, therefore the cell must expend its own metabolic energy. 3. The active transport of substances against the concentration gradient is performed by specific protein molecules embedded in the plasma membrane. 4. These transport proteins which function as carrier proteins require energy to change the shape of the protein such that the substance can be pumped across the membrane. 5. The energy required for active transport is supplied by ATP (Adenosine Triphosphate). 6. All living cells can carry out active transport. 7. An example of active transport is the pumping of sodium ions (Na+) out of the cell 8. An example of active transport is the intake of mineral ions by the root hairs of a plant. 9. In the soil, the mineral salts dissolve in water to form mineral ions. 10.The concentration of ions in the root hairs is higher than its concentration in the soil. 11. As the plant needs mineral, it has to pump the ions across the membrane of the root hairs againts the concentratin gratient. Energy is expended in the active transport of mineral ions into the root hairs of the plant. COMPARING AND CONTRASTING PASSIVE TRANSPORT AND ACTIVE TRANSPORT ASPECT PASSIVE TRANSPORT OSMOSIS DIFFUSION Movement Direction of movement Requirement of energy Requirement of special membrane proteins Selective of molecules Control by cell Example of molecules transported ACTIVE TRANSPORT FACILITATED TRANSPORT Random movement of Random movement of Random movement of molecules water molecules molecules (ions) From higher to lower concentration (Down the concentration gradient) From lower to higher concentration (Against the concentration gradient) No energy is required from the cell Uses energy from the cell (ATP molecules) No No Pore/channel proteins and carrier proteins Not selective Only water molecules Yes, very specific No No Yes Lipid-soluble molecules, gases, water Water Glucose and amino acids Selective movement of molecules (ions) Carrier proteins (also called "pumps") . Yes, very specific Yes Mineral salts, amino acids Hypotonic, Hypertonic and Isotonic Solutions Solute, Solvent, and Solution A solution is made up of two parts, the solute and the solvent. The solvent dissolves the solute. If you dissolve salt in water, you make a salt solution. The salt is the solute and the water is the solvent. The concentration of a solution refers to the quantity of solute dissolved in a unit volume of solvent. 6. The units of solution concentration are either of these units: grams per litre, grams per cubic decimetre, molarity or percentage concentration. 7. A concentrated solution has a lot of solute dissolved in the solvent. 8. A dilute solution has a small amount of solute dissolved in the solvent. 1. 2. 3. 4. 5. Hypotonic, Hypertonic and Isotonic Solutions 1. a) “Iso" means the same as, and "tonicity" refers to the strength (concentration of solute) of the solution. b) Two solutions are isotonic if they have the same solute concentrations. 2. a) “Hype”,' means more than. b) Solution A is hypertonic to solution B if solution A has a higher solute concentration than solution B. 3. a) “ Hypo" means less than. b) Solution A is hypotonic to solution B if solution A has a lower solute concentration than solution B. The Effects of Hypotonic, Hypertonic and Isotonic Solutions on Plant Cells d Animal Cells A) The effects of Hypotonic, Hypertonic and isotonic Solutions on Plant Cells 1) When a plant cell is in an isotonic solution: a) The concentration of solute in the external solution is equal to the concentration of solute in the cell sap. b) Also, the concentration of water molecules in the external solution is equal to the concentration of water molecules in the cell sap. c) Therefore, water diffuses into and out of the cell at equal rates. d) The net movement of water across the plasma membrane is zero. e) There is no change in the original size of the cell. 2. When a plant cell is in a hypotonic solution: a) The concentration of solute in the external solution is less than the concentration of solute in the cell sap. b) Also, the concentration of water molecules in the external solution is greater than the concentration of water molecules in the cell sap. c) Therefore, water molecules move into the cell by osmosis, pushing the cell contents outwards, against the cellulose cell wall and the cell becomes turgid. d) The strong and rigid cellulose cell wall pushes inwards with an equal force and prevents the cell from rupturing or bursting. e) When the cell is turgid, water molecules will diffusese into and out of the cell at equal rates (dynamic equilibrium). 3. When a plant cell is in a hypertonic solution: (a) The concentration of solute in the external solution is greater than the concentration of solute in the cell sap. (b) Also, the concentration of water molecules in the external solution is less than the concentration of water in the cell sap. (c) Therefore, water molecules diffuse out of the cell vacuole by osmosis and the cytoplasm shrinks away from the cell walL (d) Plasmolysis is the separation of plant cell cytoplasm from the cell wall as a result of water loss. (e) The plant cell becomes flaccid (soft and weak). (f) If the plasmolysed cell is placed in a hypotonic solution, it absorbs water causing the cytoplasm to expand and comes into contact with the cell wall again. This is called deplasmolysis. B) The Effects of Hypotonic, Hypertonic and Isotonic Solutions on Animal cells 1.When an animal cell is put in an isotonic solution: (a) The concentration of solute in the external solution is equal to the concentration of solute in the cell. (b) Also, the concentration of water molecules in the external solution is equal to the concentration of water molecules in the cell. (c) Therefore, water diffuses into and out of the cell at equal rates. (d) The net movement of water is zero. (e) There is no change in the size of the cell 2.When an animal cell is in a hypotonic solution: (a) The concentration of solute in the external solution is less than the concentration of the solute in the cell. (b) Also, the concentration of water molecules in the external solution is greater than the concentration of water molecules in the cell. (c) Therefore water molecules move into the cell by osmosis, inflating and finally rupturing the cell - this is called cell lysis. (d) Figure below shows the process of haemolysis, which is the rupturing of red blood cells in a hypotonic solution. 3.When an animal cell is in a hypertonic solution: (a) The concentration of solute in the external solution is greater than the concentration of solute in the cell (b) Also, the concentration of water molecules in the external solution is less than the concentration of water in the cell (c) Therefore, water molecules diffuse out of the cell by osmosis and the cell shrinks - this is called crenation (Figure below). The Effects of Hypotonic, Hypertonic and Isotonic Solutions on Plant Cells and Animal Cells