* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download An overview of Metabolism - Harford Community College
Radical (chemistry) wikipedia , lookup
Magnesium in biology wikipedia , lookup
Biochemical cascade wikipedia , lookup
Signal transduction wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Butyric acid wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Metalloprotein wikipedia , lookup
Biosynthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Photosynthesis wikipedia , lookup
Mitochondrion wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Phosphorylation wikipedia , lookup
Microbial metabolism wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Electron transport chain wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Citric acid cycle wikipedia , lookup
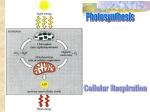
Announcements • My Pyramid extra credit project is due TODAY! • You should have turned your labs in/made up your quizzes already. • Cell/Pedigree extra credit projects are due next week. Announcements • FINAL EXAM – cumulative – 2 hrs – starts at 11am in this room – you can use a periodic table and calculator – NO CELL PHONES! Announcements • Celebrate the end of Bio 099 at the Outback Steakhouse next Saturday from 2-4pm – Here’s the address: • 615 Bel Air Rd Bel Air MD 21014 An overview of Metabolism Bio 099 December 8, 2007 Metabolism • Metabolism is all the chemical reactions that occur in a living organism. Catabolism • Catabolism is the breakdown or digestion of organic molecules. Catabolic reactions release energy in the form of ATP, which the cell can then use for its various functions. What molecules does the cell break down for energy? • Usually fats and carbohydrates are the fuel of choice – Triglycerides = • fatty acids + glycerol – Glycogen = • monosaccharides Glycogen – most abundant storage of carbohydrate – a branched chain of glucose molecules Triglycerides – most abundant storage of lipids – primarily of fatty acids Proteins – most abundant organic components in body – perform many vital cellular functions Metabolism Handout Tools for making ATP • To survive cells need to make ATP. • For ATP synthesis the following are required: – oxygen Tools for making ATP • To survive cells need to make ATP. • For ATP synthesis the following are required: – oxygen – nutrients/vitamins Tools for making ATP • To survive cells need to make ATP. • For ATP synthesis the following are required: – oxygen – nutrients/vitamins – mitochondria Tools for making ATP • To survive cells need to make ATP. • For ATP synthesis the following are required: – oxygen – nutrients/vitamins – mitochondria – enzymes Why are catabolic reactions necessary for the cell? • To release energy for anabolic reactions! Anabolism • Anabolism is the production of new organic molecules using cellular energy (ATP). For example: Proteins are produced through an anabolic reaction that uses ATP to form polypeptide bonds between amino acids. Why is anabolism necessary? 1. Metabolic Turnover: The cell needs energy to periodically replace its components. 2. Growth and Division: In order to grow and divide a cell needs energy. 3. Special Processes: Depending on the specific cell type, various functions require energy. • For example: muscle cell contraction requires energy. 4. Nutrient Pool: A cell keeps a reserve storage of nutrients, just in case… Catabolism: Aerobic Cellular Respiration Aerobic Cellular Respiration: generating ATP for the cell • Glycolysis • Krebs cycle (TCA) • Electron transport chain Aerobic Cellular Respiration • Glycolysis • Krebs cycle (TCA) • Electron transport chain happens only in the presence of oxygen aerobic: requires or takes place in the presence of oxygen Mechanisms of ATP synthesis 1. substrate-level phosphorylation occurs during glycolysis and Kreb’s cycle ADP + P ATP 2. oxidative phosphorylation occurs during the electron transport chain formation of a proton (H+) gradient across the inner mitochondrial membrane provides potential energy to make ATP Oxidation-reduction (redox) reactions are important in metabolism • Oxidation: a molecule is oxidized when it loses electrons. • Reduction: a molecule is reduced when it gains electrons. During metabolism enzymes catalyze these reactions Example of a redox reaction: NAD+ • Nicotinamide adenine dinucleotide (NAD+) is a coenzyme that carries electrons to be used in the electron transport chain. • NAD+ is made from the vitamin niacin. Example of a redox reaction: FAD+ • flavin adenine dinucleotide (FAD) is a coenzyme that carries electrons to be used in the electron transport chain. • FAD contains riboflavin (vitamin B2). Carbohydrate Catabolism • generates ATP by breaking down sugar C6H12O6 + 6O2 6H2O + 6CO2 = 36 ATP + heat 1 molecule of glucose nets 36 molecules of ATP Glucose must first get into the cell • insulin binds to its receptor to tell the cell glucose is coming and to add glucose transporter proteins to the membrane. • glucose is transported into the cell through facilitated diffusion Carbohydrate Metabolism • Glycolysis: always happens first. Glycolysis STEP 1. Hexokinase phosphorylates glucose creating glucose-6-phosphate uses 1 ATP molecule traps glucose molecule within cell Glycolysis STEP 2. Phosphoglucoisomerase transforms glucose-6-phosphate to fructose6-phophate Glycolysis STEP 3. Phosphofructokinase (PFK) adds a phosphate to fructose-6-phophate making it Fructose 1, 6 bisphosphate. This reaction requires 1 ATP. Glycolysis STEP 4. An enzyme splits Fructose 1, 6 bisphosphate into 2 glyceraldehyde-3-phosphates molecules. Glycolysis STEP 5. Each glyceraldehyde3-phosphate is oxidized to 1,3bisphosphoglycerate . At the same time NAD+ is reduced to NADH. NAD+ also donates a phosphate group in the reaction Glycolysis STEP 6. Each 1,3bisphosphoglycerate is striped of its phosphate groups making 3Phosphoglycerate. The phosphates are used to generate ATP from ADP (2 total) Glycolysis STEP 7&8. 2 more enzymatic reactions form phosphoenolypyruvate (PEP). Glycolysis STEP 9. PEP is converted to pyruvate generating ATP Glycolysis Each Glucose makes 2 molecules of glyceraldehyde phosphate so from that point on, multiply everything by 2: = 2 NADH = 4 ATP Glycolysis: Take home message 1. ATP is used in 2 reactions at the beginning of glycolysis: Glycolysis: Take home message 1. ATP is used in 2 reactions at the beginning of glycolysis: 1. to keep glucose in the cell 2. to make the molecule that is then broken in half Glycolysis: Take home message 1. ATP is used in 2 reactions at the beginning of glycolysis: 1. to keep glucose in the cell 2. to make the molecule that is then broken in half 2. 4 ATP and 2 NADH are generated in the last half of glycolysis, Glycolysis: Take home message 1. ATP is used in 2 reactions at the beginning of glycolysis: 1. to keep glucose in the cell 2. to make the molecule that is then broken in half 2. 4 ATP and 2 NADH are generated in the last half of glycolysis 3. 2 pyruvate molecules are generated from glycolysis. Carbohydrate Metabolism • Glycolysis • Pyruvic acid transition The fate of pyruvic acid • In the absence of oxygen (anaerobic) • The Electron Transport Chain (ETC) cannot run because O2 is the final electron acceptor • Because NADH2 cannot unload its H ions in the ETC it returns them to pyruvic acid forming lactate • This often happens in the muscle cells during exercise The fate of pyruvic acid • By the way… this is also how we make alcohol from sugar: fermentation The fate of pyruvic acid • In the presence of oxygen (aerobic) First, Pyruvic acid will enter the mitochondria where it is converted to acetyl CoA: The fate of pyruvic acid • In the presence of oxygen (aerobic) First, Pyruvic acid will enter the mitochondria where it is converted to acetyl CoA: 1. CO2 is removed 2. H ions leave and reduce NAD+ to NADH2 3. coenzyme A is added giving acetyl CoA The fate of pyruvic acid • In the presence of oxygen (aerobic) First, Pyruvic acid will enter the mitochondria where it is converted to acetyl CoA: 1. CO2 is removed 2. H ions leave and reduce NAD+ to NADH2 3. coenzyme A is added giving acetyl CoA Next, Acetyl CoA enters the Krebs Cycle and continues aerobic cellular respiration. Carbohydrate Metabolism • Glycolysis • Pyruvic acid transition • Kreb’s cycle Kreb’s Cycle (TCA, citric) • Acetyl CoA combines with oxaloacetic acid to form citric acid Kreb’s Cycle (TCA, citric) • Acetyl CoA combines with oxaloacetic acid to form citric acid • as the cycle continues carbons are removed, forming CO2 and NAD/FAD are reduced to NADH/FADH (electron carriers) Kreb’s Cycle (TCA, citric) • Acetyl CoA combines with oxaloacetic acid to form citric acid • as the cycle continues carbons are removed, forming CO2 and NAD/FAD are reduced to NADH/FADH (coenzymes and electron carriers) • 1 ATP molecule is made via substrate-level phosphorylation The Krebs Cycle Overall Reactants Overall Products • • • • • • • • • Acetyl-CoA 3 NAD+ FAD ADP and Pi Coenzyme A 2 CO2 3 NADH FADH2 ATP Remember: 1 glucose molecule will give double of every reactant and product! What do you get when 1 glucose molecule is broken down via aerobic respiration? • Glycolysis: – 2 ATP via substrate-level phosphorylation – 2 NADH2 • Transition Reaction (pyruvate to acetyl CoA): – 2 NADH2 • Krebs Cycle: – 6 NADH2 – 2 FADH2 – 2 ATP 36 Total ATP Metabolism Handout: • note that lipid and protein break-down also form molecules that enter the Kreb’s cycle. Electron Transport Chain (ETC) • Oxygen must be present! • Finally we will see the fate of the coenzymes (NADH, FADH2). the fate of NADH2 and FADH • NADH and FADH drop off H ions (and e-) at the ETC in the mitochondria. Electron shuttling • e- are shuttled through a sequence of membrane proteins (electron carriers). H+ pumping • this provides energy to pump H ions against their concentration gradient inner membrane matrix intermembrane space Electron carriers and H+ pumps • Two types of proteins in the inner mitochondrial membrane shuttle e- and/or pump H+. Electron carriers and H+ pumps • Two types of proteins in the inner mitochondrial membrane shuttle e- and/or pump H+. – complexes I-IV Cytochromes • Two types of proteins in the inner mitochondrial membrane shuttle e- and/or pump H+. – complexes I-IV – cytochromes • Cytochromes are proteins with heme groups that require Fe, S and Cu. Electron carriers and H+ pumps • Two types of proteins in the inner mitochondrial membrane shuttle e- and/or pump H+. – complexes I-IV – cytochromes • Cytochromes are proteins with heme groups that require Fe, S and Cu. Electron carriers and H+ pumps • Two types of proteins in the inner mitochondrial membrane shuttle e- and/or pump H+. – complexes I-IV – cytochromes • Cytochromes are proteins with heme groups that require Fe, S and Cu. • Coenzyme Q is not a protein, but still carries e-. Oxidative Phosphorylation: ADP ATP • The H+ gradient creates energy to power the ATP synthase complex. Oxidative Phosphorylation: ADP ATP • The H+ gradient creates energy to power the ATP synthase complex. • As H+ rush back into the matrix through the ATP synthase protein, ADP is phosphorlyated Oxidative Phosphorylation: ADP ATP • The H+ gradient creates energy to power the ATP synthase complex. • As H+ rush back into the matrix through the ATP synthase protein, ADP is phosphorlyated • This process is also known as chemiosmosis ETC animation Oxidative Phosphorylation: how many ATP are made? • NADH from glycolysis: – 2 ATP in electron transport chain – exception is cardiac muscle = 3 ATP in ETC • NADH from pyruvate transition reaction: – 3 ATP in electron transport chain • NADH from Kreb’s cycle: – 3 ATP in electron transport chain • FADH2 from Kreb’s cycle: – 2 ATP in electron transport chain Total ATP production from 1 molecule of glucose Total ATP production from 1 molecule of glucose Metabolism Handout: • Now we will add in the side arrows. Storing carbohydrate energy: Glycogenesis • Carried out in liver and muscle Utilizing stored energy: Glycogenolysis: • breaking down glycogen to glucose • carried out in liver making carbs from other sources: Gluconeogenesis • Formation of glucose from fatty acids and amino acids • basically glycolysis in reverse • happens in the liver Metabolism Handout: • Now we will add in the side arrows. Lipid Metabolism: digestion • fats are digested, absorbed and put into chylomicrons (large lipoprotiens). Lipid Metabolism: digestion • fats are digested, absorbed and put into chylomicrons (large lipoproteins). • Chylomicrons enter the blood stream where triglycerides are extracted. • The remnant of the chylomicron goes to the liver Metabolism Handout: • Now we will add in the side arrows. Lipid Metabolism: digestion • The triglycerides are broken down further in the blood to free fatty acids + glycerol. The fate of glycerol • glycerol is converted to glyceraldehyde-3phosphate • G-3-P enters glycolysis and then goes through Kreb’s cycle and the ETC. The fate of free fatty acids: Beta-oxidation • Fatty acids are broken down into 2 carbon acetic acid fragments. • the acetic acid is converted to acetyl-Co A, which enters the Krebs cycle and then ETC How much ATP does a 18 carbon fatty acid chain produce? Storing fat: Lipogenesis Utilizing stored energy: lipolysis • Breakdown of lipids converted to G-3-P and enters glycolysis converted to acetylCoA and enters Kreb’s cycle Ketogenesis • If you are on a low-carb diet, starving yourself, or diabetic: – oxaloacetic acid (from breakdown of glucose) levels decline and slow down the turning of the Kreb’s cycle – acetyl Co-A (from fatty acid breakdown) accumulates and the liver converts it to ketone bodies – Ketone bodies are released into the blood so they can be eliminated by the kidneys – excess ketones in blood = ketoacidosis KREB’S Metabolism Handout: • Now we will add in the side arrows. Protein Metabolism • Generally, proteins are not used for energy because we need them for protein synthesis (essential amino acids) Ammonia Urea Keto Acid pyruvate acetyl CoA Kreb’s cycle Overview of Catabolism • glucose = – glycolysis • fatty acids = – beta-oxidation • amino acids = – deamination Overview of Anabolism We talked about: • Glycogenolysis • Gluconeogenesis • Lipogenesis Cells also use ATP to make proteins and nucleic acids.