* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Addition Reactions
Woodward–Hoffmann rules wikipedia , lookup
Marcus theory wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Discodermolide wikipedia , lookup
Fischer–Tropsch process wikipedia , lookup
Elias James Corey wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
George S. Hammond wikipedia , lookup
Petasis reaction wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Ene reaction wikipedia , lookup
Stille reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Hydrogenation wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
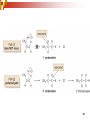
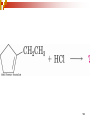
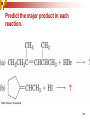
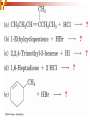
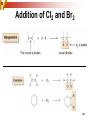
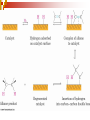
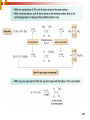
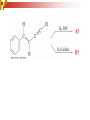
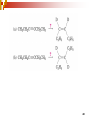
Chapter 5 Reactions of Alkenes and Alkynes Dr. Sujatha Krishnaswamy Chemistry Faculty Chandler Gilbert Community College 1 Chapter 4 Problem 18 2 Chapter 4 Problem 17 3 Addition Reactions The characteristic reaction of alkenes is addition—the bond is broken and two new bonds are formed. Because alkenes are electron rich, simple alkenes do not react with nucleophiles or bases, reagents that are themselves electron rich. Alkenes react with electrophiles. 4 5 Hydrohalogenation - Addition of HX 6 Chapter 4 Problem 14 7 To draw the products of an addition reaction: 8 Addition of HX is regioselective Markovnikov’s rule: in additions of HX, H adds to the carbon with the greater number of hydrogens 9 10 Stability of Carbocations 11 Chapter 5 Problem 3 12 13 Predict the major product in each reaction. 14 More reactions….. Chapter 5 Page 112 Problem 4 Page 116 Problem 10 15 Synthesis 17 Addition of H2O, Hydration acid-catalyzed hydration of an alkene is regioselective; hydrogen adds preferentially to the sp2 carbon with less # of hydrogens. 18 19 What products are obtained upon hydration of the following alkenes? Synthesis - How can you make these alcohols? Addition of Cl2 and Br2 22 addition is stereoselective stereoselective reaction: a reaction in which one stereoisomer is formed or destroyed in preference to all others that might be formed or destroyed addition to a cycloalkene, for example, gives only a trans product 23 Reduction of Alkenes Alkenes react with H2 in the presence of a transition metal catalyst to give alkanes the most commonly used catalysts are Pd, Pt, and Ni 24 Predict the products of hydrogenation (reduction) of Alkynes 27 Addition of HX to Alkynes 28 29 30 Addition of Halogens to Alkynes 31 Hydration of Internal Vs Terminal Alkynes Keto-enol tautomers 33 Addition of Hydrogen to Alkynes 34 Reduction of an Alkyne to an Alkane Alkane formation: 35 Addition of Hydrogen to Alkynes - Formation of cis Alkene 36 Conversion of Internal Alkynes to trans Alkenes 37 Summary of Alkyne Reductions 38 40